Abstract
Background
Despite a significant improvement of therapeutic results with the current immuno-chemotherapies, a consolidation treatment is still required for decreasing the risk of relapse.
WBRT has been the historical standard consolidation in PCNSL patients, but IC + HCR has shown encouraging results in non-controlled studies. However, each procedure exposes patients to specific side effects, such as cognitive dysfunctions and treatment-related mortality, respectively. In this trial, we addressed the efficacy and toxicity of a standard chemo-immunotherapy followed by either WBRT or IC+HCR in first-line treatment of PCNSL patients.
Methods
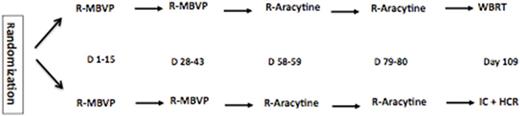
Immuno-competent patients (aged 18-60) with newly diagnosed PCNSL and measurable disease were enrolled from 23 French centers. All the patients received 2 cycles of R-MBVP (Rituximab 375 mg/m2 D1, Methotrexate 3 g/m2 D1 and D15, VP16 100 mg/m2 D2, BCNU 100 mg/m2 D3, Prednisone 60 mg/kg/D D1-D5) followed by 2 cycles of R-AraC (Rituximab 375 mg/m2 D1, Cytarabine 3g/m2 D1-D2) as induction chemotherapy. Participants were stratified upfront according to performance status (0-1 vs 2-4) and treating institution and randomly assigned (1:1)to receive either WBRT (Arm A) or IC + HCR (Arm B) as a consolidation treatment. IC consisted in thiotepa (250 mg/mg/m2/d days-9 through-7, busulfan 10 mg/kg (total dose) days-6 through -4, and cyclophosphamide 60 mg/kg/d days -3 and -2). Prospective neuro-cognitive evaluations were planned in both arms. Responses were assessed according to the IPCG criteria. A central review of MRIs was scheduled. The primary end-point was the 2-year progression free survival (PFS). Thirty-eight assessable patients who received the complete study treatment for each treatment were needed. Either of the two arms would be deemed effective if > 24/38 patients are free of disease at 2 year with no major side effects. Analysis was intent to treat, in a non-comparative phase 2 trial design.This study is registered with ClinicalTrials.gov, number NCT00863460.
Results
Between Oct 2008 and Feb 2014, 140 patients (male: 101; female: 39) were recruited (median age = 55 years, range 22-60) and randomized in Arm A (n=70) or Arm B (n = 70). Sixty-seven patients were assessable in each arm. Two patients withdrew their consent during the induction cycles (Arm A: n = 1; Arm B: n = 1). After two cycles of R-MBVP, overall response rates were 82% (CR + uCR = 24 %) and 77 % (CR + uCR = 23 %) in arm A and arm B respectively. At the end of the induction chemotherapy, ORR was 74 % (CR+ uCR = 44 %) and 67 % (CR+ uCR = 42 %) in arm A and B respectively. WBRT was given to 53 patients in arm A and IC+HCR was given to 44 patients in arm B. After consolidation treatment, ORR was 71 % (56 % CR+ Cru) and 67 % (60 % CR+ Cru) in arm A and B respectively. Five treatment-related deaths were reported, during induction chemotherapy in arm A (n = 2) and after IC+ HCT (n = 3). Median follow-up was 27.2 and 28.6 months in arm A and arm B respectively. Relapses occurred in 21 patients after the end of treatment (arm A: n = 16; Arm B: n = 5) with a median time to relapse of 15.1 and 8.5 months in arm A and B respectively. 2-y PFS in the experimental arm was 86.8% (95 CI, 76.6 to 98.3%). 2-y PFS in arm A will be given after the completion of the ongoing MRI review in this arm. The analysis of the prospective neuropsychological evaluations is pending.
Conclusion:This study shows a favorable outcome of patients who received the IC+HCR arm. Improvement of the ORR after induction chemotherapy is still needed. The results of the 2-y PFS in arm A and of the neuropsychological evaluations are awaited to define the further standard of treatment in young patients with PCNSL.
Fundings: French Government, Roche, Pierre Fabre
Choquet:Janssen: Consultancy; Celgene: Consultancy. Thyss:Takeda: Research Funding; Millennium: Research Funding. Soussain:Pharmacyclics: Research Funding; Roche: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal