Abstract
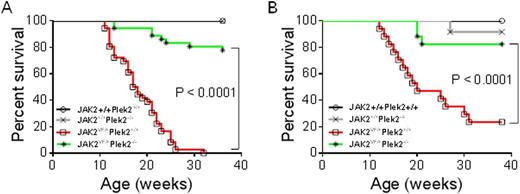
V617F driver mutation of JAK2 is the leading cause of the Philadelphia-chromosome-negative myeloproliferative neoplasms (MPNs). Studies on the pathogenesis of JAK2V617F positive MPNs have primarily focused on deregulation of JAK2 and STAT activities. It remains unclear exactly how JAK2-STAT downstream targets are involved in the development of the MPNs. We previously reported that pleckstrin-2 (Plek2) plays an important role in terminal erythropoiesis in vitro. Here we show that Plek2 is a downstream target of the JAK2-STAT5 pathway in erythroid, megakaryocytic, and granulocytic cells. Plek2 is significantly upregulated by JAK2V617F in both transduced primary mouse hematopoietic cells and in patients with JAK2V617F positive MPNs. Using a JAK2V617F knockin mouse model, we demonstrated that loss of Plek2 ameliorated JAK2V617F-induced myeloproliferative phenotypes including reticulocytosis, thrombocytosis, neutrophilia, and splenomegaly, thereby reverting the widespread vascular occlusions and lethality of JAK2V617F knockin mice (Figure 1A). These phenotypes were also transplantable indicating the role of Plek2 in mediating the pathogenesis of JAK2V617F-induced MPNs is cell-intrinsic (Figure 1B). Our study identifies Plek2 as a novel effector of JAK2-STAT5 pathway and a key factor in the pathogenesis of JAK2V617F-induced MPNs.
Loss of Plek2 Rescues The Lethality of JAK2V617F Knockin Mice. (A) Kaplan-Meier survival analysis of indicated mice. Both males and females were included in each group. JAK2+/+Plek2+/+mice, n=34; JAK2+/+Plek2-/-mice, n=34;JAK2VF/+Plek2+/+ mice, n=36; JAK2VF/+Plek2-/- mice, n=36. (B) Kaplan-Meier survival analysis of the transplanted mice. JAK2+/+Plek2+/+mice, n=10; JAK2+/+Plek2-/-mice, n=10;JAK2VF/+Plek2+/+ mice, n=17; JAK2VF/+Plek2-/- mice, n=17.
Loss of Plek2 Rescues The Lethality of JAK2V617F Knockin Mice. (A) Kaplan-Meier survival analysis of indicated mice. Both males and females were included in each group. JAK2+/+Plek2+/+mice, n=34; JAK2+/+Plek2-/-mice, n=34;JAK2VF/+Plek2+/+ mice, n=36; JAK2VF/+Plek2-/- mice, n=36. (B) Kaplan-Meier survival analysis of the transplanted mice. JAK2+/+Plek2+/+mice, n=10; JAK2+/+Plek2-/-mice, n=10;JAK2VF/+Plek2+/+ mice, n=17; JAK2VF/+Plek2-/- mice, n=17.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal