Abstract
Background: Injectable HMAs (azacitidine [AZA], decitabine) are the standard of care in higher-risk myelodysplastic syndromes (HR-MDS) (NCCN, 2016). When other treatment (Tx) options are no longer feasible, HMAs may be used to treat patients (pts) with lower-risk MDS (LR-MDS) and have demonstrated efficacy in older pts with newly diagnosed acute myeloid leukemia (AML) (Dombret, Blood, 2015; Kantarjian, JCO, 2012). Injectable HMAs induce hematologic response or improvement (HI) in ~50% of pts (Grinblatt, Haematologica, 2014; Lyons, JCO, 2009; Silverman, JCO, 2002), but responses may lack durability. There are no standard Tx options indicated for use after HMA failure; the mainstay of Tx in this setting for pts ineligible for stem cell transplant is a clinical trial or supportive care. CC-486, the oral formulation of AZA, was evaluated in 3 phase 1/2 studies that did not exclude pts who had received HMA Tx before study entry.
Aim: Determine clinical outcomes for pts with MDS, chronic myelomonocytic leukemia (CMML), or AML, treated with CC-486 monotherapy who had previously failed injectable HMA Tx.
Methods: Pts with MDS, CMML, or AML from 3 phase 1/2 CC-486 studies (2 of which included dose-finding periods) who had received HMA Tx before receiving CC-486 are included in these analyses. CC-486 Tx regimens were: 120-600mg x7 days (d) following a single SC AZA cycle (75mg/m2/d x7d), or 300mg QD or 200mg BID x14d or 21d (with no initial SC AZA cycle). All dosing regimens were administered in repeated 28d cycles. Overall response rate (ORR) included complete remission (CR), partial remission (PR), CR with incomplete hematologic recovery (CRi; AML pts only), HI, and transfusion independence (TI). Marrow CR (mCR) was assessed in MDS pts with ≥5% bone marrow blasts at baseline.
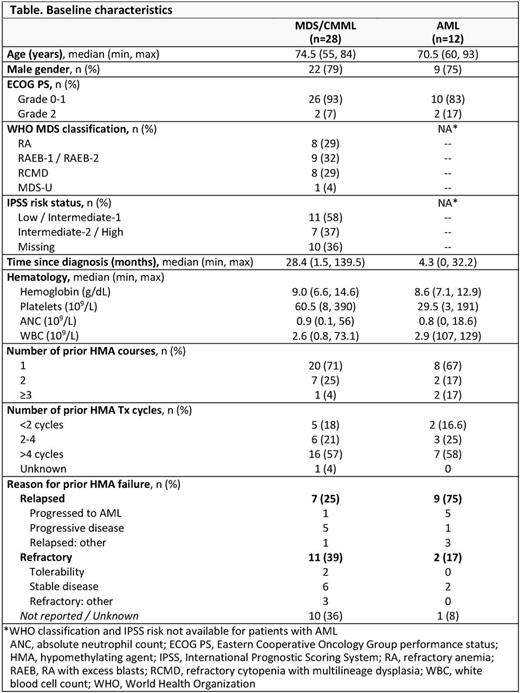
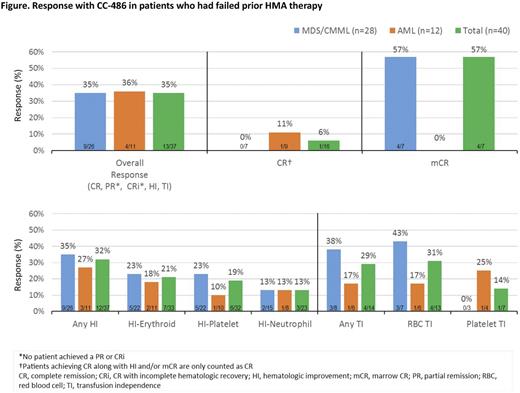
Results: In all, 40 pts had received prior HMA Tx: 26 pts with MDS, 2 with CMML, and 12 with AML. In the MDS/CMML and AML groups, respectively, median ages were 75 years (range 55-84) and 71 years (60-93) and median times since diagnosis were 28 (2-140) and 4 (0-32) months (Table). Before receiving CC-486, 12 pts (30%) had failed >1 prior injectable HMA course. Most pts (58%) had previously received >4 HMA Tx cycles. Six pts with AML (50%) had received prior HMAs for Tx of antecedent MDS. Of 29 pts for whom outcomes with prior HMAs were known, 16 pts relapsed and 13 pts were refractory to the injectable HMA. The median number of CC-486 Tx cycles was 5 (range 1-52). For all pts treated with CC-486, ORR was 35%. ORR and rates of specific responses were similar between pts with MDS/CMML and pts with AML (Figure). Five of 13 pts (38%) who were refractory to prior HMAs responded, including 1 AML pt who attained CR with CC-486. Six of 16 pts (38%) who had relapsed during or after prior HMA Tx responded. During CC-486 Tx, 32% of pts attained any HI and 31% achieved RBC TI. Of pts who had received ≥6 cycles of prior HMA Tx (n=20), ORR was 35% (7/20). ORR was not statistically different between 7d (n=26) and extended (n=14) CC-486 dosing (P=0.288). The most frequent (≥10%) grade 3-4 hematologic TEAEs were anemia (33%), thrombocytopenia (23%), neutropenia (15%), and febrile neutropenia (10%); the most frequent grade 3-4 gastrointestinal TEAEs were diarrhea and vomiting (10% each).
Conclusions: Among pts who were relapsed or refractory to prior HMA Tx, 35% had a hematologic response to CC-486, suggesting that prior HMA failure does not preclude future response to CC-486. Notably, the majority of pts had received >4 prior HMA Tx cycles; thus, prior failures to injectable HMAs were not likely due to inadequate duration of Tx. The ORR with CC-486 in pts who had received ≥6 cycles of the prior injectable HMA was the same as the ORR with CC-486 in all pts. Hypomethylating effects of HMAs are transient; unlike injectable HMAs, oral CC-486 can be administered over extended dosing periods (>7d) of the Tx cycle to produce sustained hypomethylating activity. There was no statistical difference in ORR between CC-486 7d and extended dosing in this small pt group. Nevertheless, given the short half-life and S-phase-restricted DNA incorporation of AZA (Li, Cancer Res, 1970), extending AZA exposure to >7d/cycle with CC-486 could increase the opportunity for cycling cells to incorporate AZA and expose more malignant progenitor cells to AZA, which may optimize therapeutic effects. Extended hypomethylation due to longer exposure to CC-486 may induce pts to respond to CC-486 who had failed prior injectable HMA Tx.
Savona:TG Therapeutics: Research Funding; Takeda: Research Funding; Sunesis: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Amgen Inc.: Membership on an entity's Board of Directors or advisory committees; Ariad: Membership on an entity's Board of Directors or advisory committees. Gore:Celgene: Consultancy, Honoraria, Research Funding. Scott:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding, Speakers Bureau; Alexion: Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees. Cogle:Celgene: Membership on an entity's Board of Directors or advisory committees. Conkling:USOncology Research: Research Funding; Amgen Inc.: Research Funding. Hetzer:Celgene: Employment, Equity Ownership. Dong:Celgene: Employment, Equity Ownership. Kumar:Celgene: Employment, Equity Ownership. Ukrainskyj:Celgene: Employment, Equity Ownership. Skikne:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal