Abstract
Introduction
Intensive induction chemotherapy for acute myeloid leukemia (AML) in patients aged 60 years or older has lower remission rates with increased induction mortality compared with younger patients. CPX-351 (Vyxeos) is a liposomal formulation of cytarabine and daunorubicin encapsulated at a 5:1 molar ratio. Previously reported results from a phase III, randomized, open-label study of CPX-351 versus 7+3 (cytarabine and daunorubicin) in newly diagnosed older patients with secondary AML suggested superior survival in those randomized to CPX-351. We conducted an exploratory analysis of patients who received allogeneic hematopoietic cell transplantation (HCT) after induction treatment, to determine the effect of HCT on outcome by arm, as few patients in this age range can be cured with chemotherapy alone.
Methods
This phase III trial was a randomized, open-label, parallel-arm, standard therapy-controlled study. Eligible patients were aged 60 to 75 years with newly diagnosed secondary AML defined as having a history of prior cytotoxic treatment, antecedent myelodysplastic syndrome (MDS) (± prior treatment with hypomethylating agents), or AML with World Health Organization-defined MDS-related cytogenetic abnormalities. Patients were randomized 1:1 to CPX-351 induction (100 units/m2 [100 mg/m2 cytarabine + 44 mg daunorubicin mg/m2] on days 1, 3, and 5 [first induction only]) or 7+3 induction (cytarabine 100 mg/m2/day x 7 days plus daunorubicin 60 mg/m2 on days 1, 2, and 3 [first induction] or x 5 days [reinduction/consolidation] plus daunorubicin 60 mg/m2 on days 1 and 2). The distribution of overall survival (OS) after HCT in each treatment arm was estimated using the Kaplan-Meier method, and Cox regression hazard ratio and OS rates, along with corresponding confidence intervals, are reported.
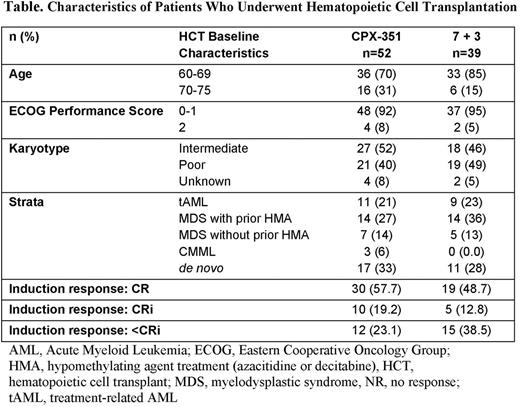
Results
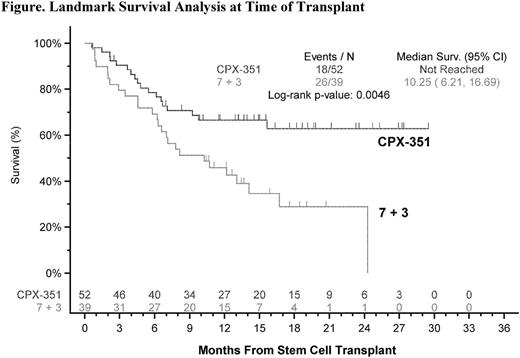
Three hundred and nine (309) patients were enrolled from December 2012 to November 2014 at 39 US and Canadian sites, with 153 patients randomized to the CPX-351 arm and 156 to the 7+3 arm. Patients in either arm responding to induction with a complete response (CR) or a CR with incomplete platelet or neutrophil recovery (n=125) were considered for allogeneic HCT when possible. In total, 91 patients were transplanted: 52 (34%) from the CPX-351 arm and 39 (25%) from the 7+3 arm. Patient and AML characteristics were similar according to randomized arm, including percentage of patients in each arm that underwent transplant in CR/CRi status (Table); however, the CPX-351 arm contained a higher percentage of older patients (age ≥ 70) who were transplanted (CPX-351, 31%; 7+3, 15%). Mortality at 100 days after transplant was 9.6% for patients in the CPX-351 arm and 20.5% in the 7+3 arm patients. Causes of death <100 days post-HCT were refractory AML (CPX-351, 3.8%; 7+3, 7.7%), graft-vs-host disease (CPX-351, 3.8%; 7+3, 2.6%), renal, respiratory, multi-organ failure, or septic shock (CPX-351, 0 for each; 7+3, 2.6% for each), unknown (CPX-351, 1.9%; 7+3, 0). Kaplan-Meier analysis of the 91 transplanted patients landmarked at the time of stem-cell transplant showed that patients in the CPX-351 arm had markedly better OS (hazard ratio 0.46; P=.0046; Figure). The time-dependent Cox hazard ratio for OS the CPX-351 arm versus the 7+3 arm was 0.51 (95% confidence interval, 0.35-0.75; P=.0007).
Conclusions
An exploratory analysis from this phase III study demonstrated that CPX-351, compared with standard cytarabine and daunorubicin, resulted in better outcomes after allogeneic HCT in older patients with high-risk AML, including 53% fewer deaths within 100 days of transplant. These results suggest that CPX-351 may provide an effective bridge to successful transplant for a very poor-risk subgroup of AML patients.
Support: Celator Pharmaceuticals, Inc., a subsidiary of Jazz Pharmaceuticals plc.
Uy:Boehringer Ingelheim: Consultancy; Glycomimetics: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; Bristol-Myers Squib: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Ritchie:Astellas Pharma: Research Funding; Ariad: Speakers Bureau; Celgene: Consultancy, Other: Travel, Accomodations, Expenses, Speakers Bureau; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; NS Pharma: Research Funding; Bristol-Meyers Squibb: Research Funding. Stuart:Celator: Research Funding; Incyte: Research Funding; Astellas: Research Funding; Sunesis: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding; Agios: Research Funding; Bayer: Research Funding. Strickland:Alexion Pharmaceuticals: Consultancy; Ambit: Consultancy; Baxalta: Consultancy; Boehringer Ingelheim: Consultancy, Research Funding; CTI Biopharma: Consultancy; Daiichi Sankyo: Consultancy; Sunesis Pharmaceuticals: Consultancy, Research Funding; Abbvie: Research Funding; Astellas Pharma: Research Funding; Celator: Research Funding; Cyclacel: Research Funding; GlaxoSmithKline: Research Funding; Karyopharm Therapeutica: Research Funding; Sanofi: Research Funding. Hogge:Sanofi: Consultancy; Roche: Other: Travel, Accomodations, Expenses. Stone:Sunesis Pharmaceuticals: Consultancy; Xenetic Biosciences: Consultancy; Agios: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celator: Consultancy; Roche: Consultancy; Seattle Genetics: Consultancy; Juno Therapeutics: Consultancy; Jansen: Consultancy; Merck: Consultancy; ONO: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy. Kolitz:Gliead Sciences: Consultancy; Pharmacyclics: Consultancy; Seattle Genetics: Consultancy. Schiller:Celator: Research Funding. Ryan:AbbVie: Equity Ownership; U of Rochester: Patents & Royalties. Chiarella:Celator Pharmaceuticals, Inc., a subsidiary of Jazz Pharmaceuticals plc.: Employment, Equity Ownership. Louie:Celator Pharmaceuticals, Inc., a subsidiary of Jazz Pharmaceuticals plc.: Employment, Equity Ownership. Medeiros:MEI Pharma: Research Funding; Merck/Schering Plough: Research Funding; Celgene: Consultancy, Other: Travel, Accomodations, Expenses, Research Funding; ARIAD: Consultancy; Celator: Other: Travel, Accomodations, Expenses, Research Funding; Roche/Genentech: Consultancy, Research Funding; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal