Abstract
Introduction
The myeloproliferative neoplasms (MPNs) are clonal stem cell disorders characterized by hematopoietic stem/progenitor cell (HSPC) expansion and overproduction of mature blood cells. The acquired mutation JAK2V617F plays a central role in these disorders. The mechanisms responsible for MPN HSPC expansion are not fully understood, limiting the effectiveness of current treatments. Endothelial cells (ECs) carrying the JAK2V617F mutation can be detected in patients with MPNs. In this work we test the hypothesis that the JAK2V617F-mutant vascular niche promotes JAK2V617F clonal expansion in MPNs.
Methods
JAK2V617F Flip-Flop (FF1) mice (Radek Skoda, Switzerland) and Tie2-Cre mice (Mark Ginsberg, UC San Diego) were crossed to generate a strain in which human JAK2V617F is expressed specifically in hematopoietic cells and ECs (Tie2/FF1). Animal experiments were performed in accordance with the Institutional Animal Care and Use Committee guideline.
Results
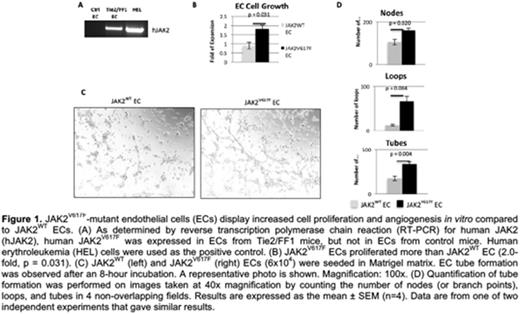
Primary lung ECs (CD45-CD31+) were isolated from Tie2/FF1 mice (JAK2V617F ECs) and age-matched littermate control mice (JAK2WT EC). JAK2V617F ECs proliferated to a greater extent than JAK2WT ECs. A vascular tube formation assay was performed as a measure of in vitro angiogenesis and revealed that JAK2V617F ECs had significantly increased angiogenesis compared to JAK2WT EC. (Figure 1)
To examine the role of JAK2WT and JAK2V617F ECs in MPN hematopoiesis in vitro, marrow Lin-cKit+ HSPCs were isolated from Tie2/FF1 and age-matched littermate controls and cultured on a feeder layer of JAK2WT or JAK2V617F ECs under serum-free conditions. While there was no difference between JAK2V617F and JAK2WT HSPC proliferation when co-cultured with JAK2WT EC, the JAK2V617F HSPC displayed a relative growth advantage over the JAK2WT HSPC (1.24-fold, p = 0.041) when co-cultured on JAK2V617F EC.
Next, the effects of JAK2V617F-bearing vascular niche on MPN hematopoiesis were studied in vivo using marrow transplantation assays. First, wild-type CD45.1 marrow cells were transplanted into lethally irradiated (950cGy) 8-14 week old Tie2/FF1 mice or controls (CD45.2) (n=7 in each group). During a >8-month follow up, all wild-type recipients displayed full donor engraftment, while 4 of 7 Tie2/FF1 recipient mice displayed recovery of JAK2V617F-mutant hematopoiesis (mixed donor/recipient chimerism), suggesting that the JAK2V617F-mutant vascular niche may protect the JAK2V617F-mutant HSPCs from the lethal irradiation. Although marrow and splenic hematopoietic progenitors were significantly increased in the Tie2/FF1 recipients, there was no difference in peripheral blood (PB) cell count or PB hematopoietic progenitor cell numbers between the Tie2/FF1 mice and control mice. (Figure 2)
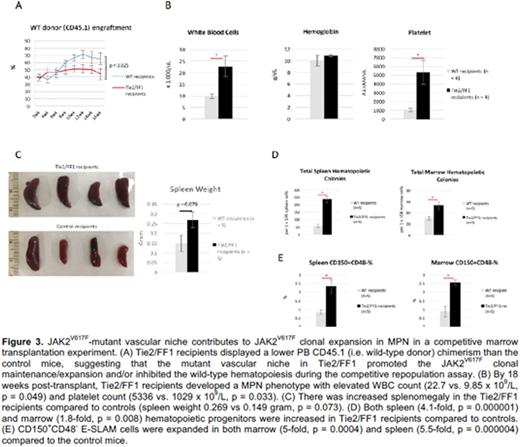
Second, we performed a competitive marrow transplantation experiment in which 5 x 105 CD45.2 donor marrow cells from Tie2/FF1 mice were injected intravenously together with 5 x 105 competitor CD45.1 wild-type marrow cells intro lethally irradiated Tie2/FF1 mice or control mice (CD45.2) (n=5 in each group). During a 4-month follow up, Tie2/FF1 recipients displayed lower PB CD45.1 (i.e. wild-type donor) chimerism than the control mice (p = 0.025), suggesting that the mutant vascular niche in Tie2/FF1 promoted the JAK2V617F clonal maintenance/expansion and/or inhibited the wild-type hematopoiesis. By 18 weeks post-transplant, Tie2/FF1 recipients developed a MPN phenotype with elevated WBC count (22.7 vs. 9.85 x 109/L, p = 0.049), platelet count (5336 vs. 1029 x 109/L, p = 0.033), increased splenomegaly, increased total marrow and splenic hematopoietic progenitors, and increased CD150+CD48- E-SLAM cells (a highly enriched HSPC population) in both marrow (5-fold, p = 0.0004) and spleen (5.5-fold, p = 0.000004) compared to the control mice. To begin to understand the EC signals responsible for JAK2V617F clonal expansion and MPN pathogenesis, qRT-PCR of purified marrow ECs identified up-regulation of MPL and CXCL12 in both arterial (CD45-CD31+Sca1+) and sinusoidal (CD45-CD31+Sca1-) marrow ECs in Tie2/FF1 mice compared to controls.
Conclusions
Our study establishes that JAK2V617F-bearing ECs form an important part of the MPN HSPC niche and contribute to mutant stem/progenitor cell expansion both in vitro and in vivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal