Abstract
The identification of mutations in BRAFV600E and other kinases in systemic histiocytoses changed our understanding of the pathophysiology of these disorders from inflammatory non-malignant conditions to clonal disorders driven by altered MAP kinase signaling. In addition, BRAFV600E mutations have recently been noted in bone marrow (BM) CD34+ cells of patients with Langerhans Cell Histiocytosis (LCH), suggesting that LCH represents a clonal disorder arising from hematopoietic stem/progenitor cells (HSPCs). However, it is unknown if HSPCs from LCH or other histiocytoses patients can functionally give rise to these disorders. Moreover, whether or not patients with histiocytosis have additional hematological disorders related to the presence of HSPC-based mutations is unclear. Here we attempted to understand the spectrum of hematological disorders in patients with histiocytoses and the cell-of-origin of histiocytic neoplasms by functionally analyzing HSPCs from histiocytoses patients.
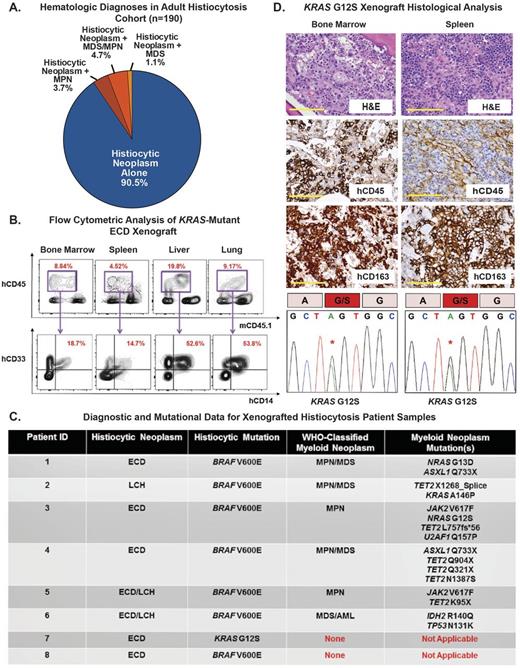
We first analyzed a multicenter cohort of 190 adult histiocytoses patients. This unexpectedly identified a high frequency of patients with a co-existing myeloid malignancy. Of the 190 patients, 9.5% (18/190) were diagnosed with Erdheim-Chester Disease (ECD) and/or LCH concomitantly with a classic MPN (3.7%; 7/190), MPN/MDS overlap (4.7%; 9/190), or MDS (1.1%; 2/190) (Figure). This clinical observation was confirmed by detection of co-existing histiocytoses-associated kinase mutations (including BRAF, MAP2K1, NRAS, or KRAS mutations) with JAK2 or CALR mutations, as well as other mutations recurrently found in myeloid malignancies (such as IDH1/2, ASXL1, and TET2 mutations (Figure)). These data therefore reveal a heretofore-unrecognized clinical overlap of adult-onset systemic histiocytoses and WHO-classified myeloid malignancies while further highlighting the possibility of an aberrant HSPC origin for histiocytic neoplasms. In most cases, the BRAF/MAP2K1/RAS mutation predominated in the histiocytic lesion while the JAK2/CALR mutation predominated in the BM/peripheral blood suggesting likely distinct clones driving each clinical condition.
Next, to test the self-renewal and differentiation capacity of HSPCs from histiocytoses patients, CD34+ cells were purified from 8 patients including 6 with ECD or LCH plus a concomitant myeloid neoplasm, and 2 with ECD alone. 0.1-0.8x106 CD34+ cells from each patient were transplanted by intrafemoral injection into sublethally irradiated NOD/SCID IL2Rγ transgenic human GM-CSF, IL-3, and SCF expressing (NSG-SGM3) mice. Human engraftment was monitored by monthly flow cytometric analysis of peripheral blood until mice became moribund. Thereafter, animals were sacrificed and tissues were analyzed using flow cytometry, histology, immunohistochemistry, and sequencing analyses to detect driver mutations identified in the histiocytosis/co-occurring myeloid neoplasm.
Successful engraftment of human myeloid cells was verified in grafts generated from 2/8 patients after a mean of 90 days (range: 60-120 days). This included lethal engraftment of KRAS-mutated ECD patient cells at 90 days manifested by infiltration of human hCD45+ cells co-expressing human myeloid and monocyte lineage markers hCD33 and hCD14 in the bone marrow, spleen, liver, lung, and kidney (Figure). Immunostains revealed that tissues were infiltrated by an hCD45+hCD163+hCD68+ population of foamy histiocytes, characteristic of ECD (Figure). Furthermore, genomic analysis of DNA from the engrafted animals BM and spleen revealed the same KRASG12S mutation found in the donor patients ECD lesion. Engraftment of a patient diagnosed with concomitant BRAFV600E-mutant ECD and ASXL1/TET2-mutant CMML was seen at 60 days. In this case, however, only engraftment of human CMML was detected without evidence of the BRAFV600E-mutant ECD component.
This study suggests that adult histiocytic neoplasms frequently co-occur with other myeloid neoplasms. Moreover, histiocytosis disease-driver mutations are present in the CD34+ HSPCs, which we show for the first time can initiate disease that resembles human histiocytosis in a patient-derived xenograft. Importantly, however, even though the CD34+ compartment gives rise to both the histiocytic and myeloid neoplasm disease component, each disease is likely initiated by distinct precursors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal