Abstract
Background: The Hedgehog signaling pathway (HhP) is aberrantly activated in leukemias and myelodysplastic syndrome (MDS), promoting cancer stem cell maintenance. HhP inhibition reduces leukemic stem cells. Glasdegib is a potent, selective, oral HhP inhibitor, with activity in pre-clinical and clinical studies. The addition of glasdegib to standard chemotherapy (CT) has an acceptable safety profile and appears to have clinical activity in MDS and acute myeloid leukemia (AML).
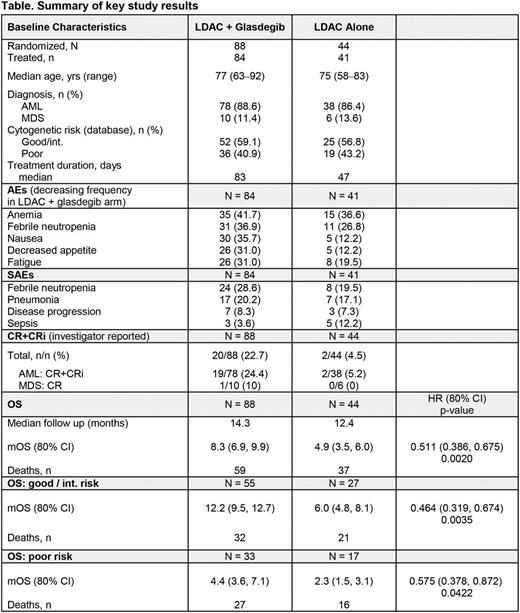
Methods: In this study (NCT01546038), previously untreated AML or high-risk MDS patients (pts) ineligible for intensive CT were randomized 2:1 to receive low-dose cytarabine (LDAC) 20 mg subcutaneously twice a day x 10 days q28 days + oral glasdegib 100 mg daily or LDAC alone for as long as pts received clinical benefit. The primary endpoint was overall survival (OS). The final analysis was conducted after completion of recruitment (Oct 2015) and at least 92 OS events.
Results: As of Apr 2016, 132 pts (116 AML, 16 MDS) were randomized to LDAC + glasdegib (n = 88) or LDAC alone (n = 44) (stratified as good/intermediate [int.] vs poor risk) (Table). Demographic and baseline characteristics were similar between arms in median age, baseline cytogenetic risk, and diagnosis. Eighty-four pts received LDAC + glasdegib and 41 pts LDAC alone (7 randomized/not treated pts were followed for survival). Median treatment duration was 83 days for LDAC + glasdegib and 47 days for LDAC alone; median follow up was 14.3 months and 12.4 months, respectively. In the glasdegib arm, 12 pts were continuing treatment and 25 were in follow up; in the LDAC arm, 1 pt was on treatment and 5 in follow up. Cytopenias and gastrointestinal toxicities were the adverse events (AEs) occurring more frequently in the LDAC + glasdegib arm. Hh-associated AEs in the glasdegib arm included dysgeusia (23.8%), muscle spasms (20.2%) and alopecia (10.7%). Serious AEs of febrile neutropenia were more frequent in the glasdegib arm, but sepsis rates were lower and pneumonia rates were similar. The most common cause of death was disease progression in both arms. Grade 2-4 QTcF prolongation was more frequent in the LDAC arm.
Investigator-reported complete response (CR) rates were numerically higher for LDAC + glasdegib (n = 17, 15%) vs LDAC alone (n = 1, 2.3%), p-value 0.0142. Based on intent to treat analysis of 96 events, median OS (mOS) for LDAC + glasdegib was 8.3 (80% confidence interval [CI] 6.9, 9.9) vs 4.9 months (80% CI 3.5, 6.0) for LDAC alone (HR 0.511, 80% CI 0.386, 0.675; one-sided log rank p-value 0.0020 stratified by cytogenetic risk). For good/int. risk, mOS for LDAC + glasdegib was 12.2 vs 6.0 months for LDAC alone (HR 0.464, p-value 0.0035). For poor risk, mOS for LDAC + glasdegib was 4.4 vs 2.3 months (HR 0.575, p-value 0.0422). In AML pts, mOS for LDAC + glasdegib was 8.3 vs 4.3 months for LDAC alone (HR 0.462, p-value 0.0004).
Conclusions: The addition of glasdegib to LDAC for AML and high-risk MDS pts improved OS compared with LDAC alone. The improvement was consistent among subgroups, particularly in good/int. risk pts. Treatment was associated with an acceptable safety profile. The addition of glasdegib to LDAC may be a treatment option for pts with AML or high-risk MDS.
Cortes:ARIAD: Consultancy, Research Funding; Bristol-Myers Squib: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Heuser:Tetralogic: Research Funding; Celgene: Honoraria; Bayer Pharma AG: Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; Karyopharm Therapeutics Inc: Research Funding; BerGenBio: Research Funding. Fiedler:Gilead: Other: Travel; Novartis: Consultancy; Ariad/Incyte: Consultancy; Teva: Other: Travel; Pfizer: Research Funding; Kolltan: Research Funding; Amgen: Consultancy, Other: Travel, Patents & Royalties, Research Funding; GSO: Other: Travel. Smith:Actinium Pharmaceuticals, Inc.: Research Funding. Robak:Pfizer: Research Funding. Montesinos Fernandez:Gamida Cell: Consultancy. Ma:Pfizer: Employment, Equity Ownership. Shaik:Pfizer: Employment, Equity Ownership. Zeremski:Pfizer: Employment, Equity Ownership. O'Connell:Pfizer: Employment, Equity Ownership. Chan:Pfizer: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal