Abstract
Atrial fibrillation is the most common cardiac arrhythmia and conveys a significant risk of morbidity and mortality due to related stroke and systemic embolism. Oral anticoagulation (OAC) is the mainstay of thromboembolism prevention, and management of anticoagulation can be challenging. For patients without significant valvular disease, decisions around anticoagulation therapy are first based on the presence of additional stroke risk factors, as measured by the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75, diabetes, prior stroke or transient ischemic attack, vascular disease, age 65–74, and sex category [female]) score. Patients with increased CHA2DS2-VASc scores (by regional guidelines) should next be evaluated to determine if they are candidates for non–vitamin K antagonist oral anticoagulant (NOAC) therapy. This should focus on assessment of concomitant valve disease and/or impaired renal function. In eligible patients, the cumulative data support a preference for NOACs over warfarin, as NOACs appear safer and more effective as a group. However, there are no direct, randomized comparisons between NOACs, and therefore, selecting among them can be difficult. In addition, important patient groups remain underrepresented in major clinical trials, and their management is often left to clinician judgment. Data from emerging clinical trials will help guide physicians; however, patient engagement in decisions regarding OAC management will remain vital to ensuring appropriate balance of risks and optimizing health outcomes.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac dysrhythmia worldwide, and its prevalence continues to grow.1 In addition to causing significant symptom burden, AF is a source of major morbidity and mortality due to its accordant risk of stroke.2,3 Although the complete causative pathways behind AF-related stroke continue to be the source of investigation, cardioembolism appears to be a major factor.4,5 Following several major trials and meta-analyses, oral anticoagulation (OAC) was found to be highly effective at reducing stroke and mortality in patients with nonvalvular AF at the expense of increased bleeding risk.6 In appropriately selected patients, OAC has become the standard of care for stroke prevention in AF worldwide.7,8
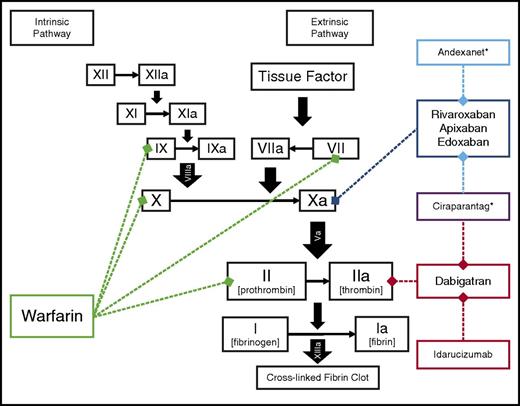
The evidence supporting guideline recommendations for OAC was primarily based on trials of dose-adjusted vitamin K antagonism (ie, warfarin), despite its inherent shortcomings of long half-life, variable dosing, and numerous food and drug interactions. In 2010, the first non–vitamin K antagonist oral anticoagulant (NOAC) was approved: the direct-thrombin inhibitor, dabigatran. Since 2010, 3 additional NOACs have been approved for stroke prevention in AF, in yet another class: the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban (Figure 1). Physicians treating patients with AF now find themselves with an embarrassment of riches: where historically there was only 1 choice for OAC, now they must identify the best choice among several. The present review discusses the decision process for selecting among stroke-prevention strategies in patients with AF and highlights several specific, challenging clinical scenarios.
Sites of action for oral anticoagulants approved for stroke prevention in AF. Idarucizumab, andexanet,* and ciraparantag* are specific reversal agents for the NOAC.7,33,66 *Not currently approved for clinical use.
Overview of treatment
Candidates for treatment
Anticoagulation is not recommended for all patients with AF, only those in which the risk of bleeding with OAC treatment is outweighed by the benefit of stroke risk reduction. Historically, the highest-risk patients with AF were those with valvular AF, ie, patients with rheumatic mitral stenosis. These patients are at such a high risk of thromboembolism that they were not included in most of the original trials of warfarin for stroke prevention in AF because it was felt to be unethical to withhold anticoagulation in this setting. Furthermore, patients with valvular disease (by varying definitions) were also excluded from the more contemporary trials of NOACs for stroke prevention. Last, the 1 trial of NOAC therapy in patients with mechanical heart valves was terminated early due to a signal of harm in the NOAC group.9 For these reasons, warfarin remains the only choice for patients with valvular AF and/or mechanical heart valves.7 The remaining text focuses on patients with nonvalvular AF, which has been a challenge for physicians to define consistently.10 Among the major, recent trials of nonvalvular AF, there has been significant heterogeneity, which has led many physicians to simplify the definition to include all patients without a mechanical valve replacement and without moderate or severe rheumatic mitral valve disease.11 Nevertheless, the most recent American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for AF management use a more limited definition: patients without rheumatic mitral stenosis, prior mitral valve surgery, or any valve replacement.7
Among patients with nonvalvular AF, stroke prevention with OAC is indicated for those at particularly increased risk of stroke. Risk of stroke was historically defined using the CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack [2 points]) scoring algorithm (a score ≥2 warranting therapy),12 and more contemporary trials have used the refined CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 [2 points], diabetes, prior stroke or transient ischemic attack [2 points], vascular disease, age 65–74, and sex category [female]) scoring algorithm (Table 1).13 Although the CHA2DS2-VASc algorithm was developed in an effort to better discriminate patients of truly low risk versus intermediate or high risk, it is important to acknowledge the limitations of these scores: the derivation cohorts were relatively small, yielding wide confidence intervals around the event rate estimates due to very low numbers of events in the lowest risk cohorts.12,13 Nevertheless, the CHA2DS2-VASc scoring algorithm has replaced the CHADS2 score as the primary stroke risk stratification tool in major international guidelines for AF.7,8 Pivotal randomized, clinical trials have demonstrated benefit of OAC therapy in patients with CHA2DS2-VASc score ≥2. Although this has been the benchmark maintained in the most recent American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines, the latest European guidelines have taken a more nuanced approach.14 Based on data suggesting female sex alone to be a very weak risk factor for stroke, these guidelines recommend OAC for men with a CHA2DS2-VASc score of ≥2 and women with a score ≥3; they suggest OAC be considered for men with CHA2DS2-VASc of ≥1 and women with a score ≥2.
Empirical risk scores for stroke and bleeding risk in AF
| Stroke risk scores . | Bleeding risk scores . |
|---|---|
| CHADS212 | ATRIA15 |
| Congestive heart failure (1 point) | Anemia (3 points) |
| Hypertension (1 point) | Severe renal disease (3 points) |
| Age ≥75 (1 point) | Age ≥75 (2 points) |
| Diabetes (1 point) | Prior bleeding (1 point) |
| Stroke or TIA (2 points) | Hypertension (1 point) |
| CHA2DS2-VASc13 | HAS-BLED16 |
| Congestive heart failure (1 point) | Hypertension (1 point) |
| Hypertension (1 point) | Abnormal liver or renal function (1 point each) |
| Age ≥75 (2 points) | Stroke (1 point) |
| Diabetes (1 point) | Bleeding (1 point) |
| Stroke or TIA (2 points) | Labile international normalized ratio (1 point) |
| Vascular disease (1 point) | Elderly: age >65 (1 point) |
| Age 65-74 (1 point) | Drugs or alcohol (1 point each) |
| Sex category (1 point for female, 0 for male) | |
| ORBIT17 | |
| Older age (1 point) | |
| Reduced hemoglobin/anemia (2 points) | |
| Bleeding history (2 points) | |
| Insufficient kidney function (1 point) | |
| Treatment with antiplatelet |
| Stroke risk scores . | Bleeding risk scores . |
|---|---|
| CHADS212 | ATRIA15 |
| Congestive heart failure (1 point) | Anemia (3 points) |
| Hypertension (1 point) | Severe renal disease (3 points) |
| Age ≥75 (1 point) | Age ≥75 (2 points) |
| Diabetes (1 point) | Prior bleeding (1 point) |
| Stroke or TIA (2 points) | Hypertension (1 point) |
| CHA2DS2-VASc13 | HAS-BLED16 |
| Congestive heart failure (1 point) | Hypertension (1 point) |
| Hypertension (1 point) | Abnormal liver or renal function (1 point each) |
| Age ≥75 (2 points) | Stroke (1 point) |
| Diabetes (1 point) | Bleeding (1 point) |
| Stroke or TIA (2 points) | Labile international normalized ratio (1 point) |
| Vascular disease (1 point) | Elderly: age >65 (1 point) |
| Age 65-74 (1 point) | Drugs or alcohol (1 point each) |
| Sex category (1 point for female, 0 for male) | |
| ORBIT17 | |
| Older age (1 point) | |
| Reduced hemoglobin/anemia (2 points) | |
| Bleeding history (2 points) | |
| Insufficient kidney function (1 point) | |
| Treatment with antiplatelet |
Modified from Steinberg et al.67
In contrast to stroke risk scores, assessing risk of bleeding with OAC is more complicated. Nevertheless, it remains a vital step in the selection of appropriate therapy for stroke prevention in AF. All OAC therapies increase risk of bleeding, and thus, the net clinical benefit of OAC therapy is tied to both the risk reduction of stroke and the incremental increased risk of bleeding on treatment. Several simplified scores have been developed to assess patient bleeding risk in the setting of OAC (Table 1), and they have been tested in several different populations.15-17 Although these scores can be helpful to clinicians treating AF patients, there are 2 major caveats: (1) in contrast to the data supporting OAC based on stroke risk score, there have been no randomized clinical trials demonstrating the benefit of withholding OAC in AF patients on the basis of a bleeding risk score—physicians are left to judge for themselves if there is an appropriate cutoff; and (2) large, observational data have suggested persistent net clinical benefit of OAC even among patients of very high score-based bleeding risk.18 The persistent net clinical benefit is likely due in part to the significant overlap between stroke and bleeding risk factors: patients at increased risk of bleeding are very commonly also at increased risk of stroke. Therefore, although guidelines acknowledge the availability of bleeding risk scores, the recommendations for their use are more tempered than those for stroke risk scores.7,8 They are important tools to help remind clinicians of bleed risk factors, and particularly, to highlight management of reversible risk factors for bleeding. For example, the concomitant use of aspirin and/or nonsteroidal anti-inflammatory medications increases bleeding risk with OAC and should be discontinued when not clinically indicated.19 In addition, careful attention to blood pressure control can reduce the risk of stroke and intracranial hemorrhage (ICH).
Selection of an anticoagulant
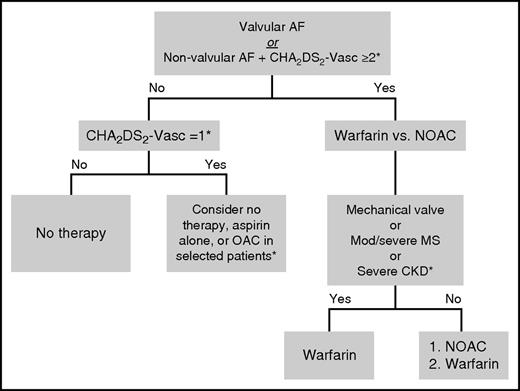
Among patients with nonvalvular AF and stroke risk sufficient to warrant OAC, selection of an agent can be challenging (Figure 2). Determining whether an NOAC is contraindicated for a patient is an important step; in addition to patients with valvular disease, this also includes additional, specific patient groups. First, patients with severe liver disease (eg, Child–Pugh B or C, liver function tests persistently >2 times the upper limit of normal, or active viral hepatitis) are at increased risk for bleeding at baseline; although warfarin can be used and carefully monitored in these patients, there are very little data to support the safe use of NOACs in these patients. Second, patients with severe chronic kidney disease in the presence or absence of renal replacement therapy; although some of the package inserts for NOACs provide dosing guidance in this group, it is based predominantly on small pharmacokinetic studies, and these high-risk patients were not included in the large, pivotal, clinical trials. For those reasons, many clinicians are not comfortable routinely using these drugs in this population. Third, there are patients who cannot afford NOACs or have difficulty adhering to daily medical regimens. Dose-adjusted warfarin may be preferred in these patients, as inconsistent compliance with OAC can be dangerous. The adverse effects of missed doses may be particularly pronounced for NOACs, as their half-lives are much shorter compared with warfarin. The readily available test for the therapeutic effect of warfarin may make it preferred in some patients (eg, those in whom compliance or targeted drug effect are likely to be inconsistent).
Selection of OAC for stroke prevention in patients with AF and without a contraindication to systemic anticoagulation. *Subject to interpretation of guidelines and prescribing information; the latest US and European guidelines are not consistent with regards to CHA2DS2-VASc score, and this figure primarily reflects the US recommendations. See text for details. CKD, chronic kidney disease; MS, mitral stenosis.
Selection of OAC for stroke prevention in patients with AF and without a contraindication to systemic anticoagulation. *Subject to interpretation of guidelines and prescribing information; the latest US and European guidelines are not consistent with regards to CHA2DS2-VASc score, and this figure primarily reflects the US recommendations. See text for details. CKD, chronic kidney disease; MS, mitral stenosis.
For patients who are candidates for an NOAC, current clinical evidence supports the preferential use of NOACs over warfarin: each of the major clinical trials demonstrated a minimum of noninferiority for both bleeding and stroke prevention,20-23 and the major meta-analysis of NOACs versus warfarin demonstrated consistent overall benefits in terms of safety and efficacy for NOACs.24 In addition, the European AF guidelines currently favor NOACs over warfarin, where available.8,14 However, selection among NOACs remains a significant clinical dilemma: there have been no randomized, head-to-head, direct comparisons between any of these agents for stroke prevention in AF. Although there are many smaller, observational, and/or indirect comparisons, none provides a comprehensive evaluation. However, there are some important factors to consider when deciding among the NOACs. The first often is still financial: many US payers will have a preferred agent for their patients, and given the lack of compelling data favoring one over another, this is often not unreasonable.
In the patient with multiple NOACs available, there may be clinical considerations in choosing among them.25-28 First, there are some important differences among the pivotal clinical trials of these drugs, which may influence agent selection in a particular patient (Table 2). The Randomized Evaluation of Long-Term Anticoagulation Therapy trial of dabigatran was the only one to allow the concomitant use of “triple” therapy, that is, OAC plus DAPT with aspirin and a P2Y12 inhibitor (ie, clopidogrel).28,29 Use of DAPT consistently increases risk of bleeding, but did not influence the benefit of dabigatran over warfarin. The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism trial of rivaroxaban enrolled the population with the highest stroke risk, as measured by CHADS2 score. Apixaban is the only agent studied in a prospective, randomized, clinical trial comparing NOAC with aspirin for stroke prevention in patients unsuitable for warfarin; this demonstrated superior stroke prevention with no significant increase in bleeding risk (formal noninferiority testing was not performed).30 The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 trial of edoxaban tested the most structured protocol for transition between an NOAC and warfarin.23
Selected distinctions among pivotal randomized controlled trials of NOAC for stroke prevention in nonvalvular in AF
| . | RE-LY20 . | ROCKET-AF21 . | ARISTOTLE22 . | ENGAGE AF-TIMI 4823 . | AVERROES30 . |
|---|---|---|---|---|---|
| Comparison | Dabigatran twice daily (110 mg, 150 mg)* vs warfarin (open-label) | Rivaroxaban 20 mg daily vs warfarin | Apixaban 5 mg twice daily vs warfarin | Edoxaban daily (30 mg, 60 mg)* vs warfarin | Apixaban 5 mg twice daily vs aspirin |
| No. of patients (approx.) | 18 000 | 14 000 | 18 000 | 21 000 | 5600 |
| Valve exclusion | Severe valve disorder | Significant MS; any prosthesis | Mod/sev MS; mechanical | Mod/sev MS; mechanical | Requires surgery; mechanical |
| Renal exclusion | CrCl < 30 mL/min | CrCl < 30 mL/min | Cr > 2.5 mg/dL or CrCl < 25 mL/min | CrCl < 30 mL/min | Cr > 2.5 mg/dL or CrCl < 25 mL/min |
| Protocol-specified dose-reduction for renal impairment | None | CrCl 15-50 mL/min: 15 mg daily | ≥2 of following age >80, weight <60 kg, Cr >1.5 mg/dL (2.5 mg twice daily) | ≥1 of the following: CrCl 30-50 mL/min, weight ≤60 kg, concomitant use of potent P-glycoprotein inhibitors (halved dose) | ≥2 of following age >80, weight <60 kg, Cr >1.5 mg/dL (2.5 mg twice daily) |
| CHADS2, mean | 2.1 | 3.5 | 2.1 | 2.8 | 2.0 |
| Triple therapy allowed (OAC+DAPT)28 | Yes | No | No | No | No |
| Stroke outcomes | Noninferior (110 mg), superior (150 mg) | Noninferior | Superior | Noninferior | Superior |
| Bleeding outcomes | Superior (110 mg), noninferior (150 mg) | Noninferior (ICH/fatal vs GI) | Superior | Superior | Not significantly different |
| All-cause mortality reduced | No | No | Yes | Yes (30 mg), | No |
| No (60 mg) |
| . | RE-LY20 . | ROCKET-AF21 . | ARISTOTLE22 . | ENGAGE AF-TIMI 4823 . | AVERROES30 . |
|---|---|---|---|---|---|
| Comparison | Dabigatran twice daily (110 mg, 150 mg)* vs warfarin (open-label) | Rivaroxaban 20 mg daily vs warfarin | Apixaban 5 mg twice daily vs warfarin | Edoxaban daily (30 mg, 60 mg)* vs warfarin | Apixaban 5 mg twice daily vs aspirin |
| No. of patients (approx.) | 18 000 | 14 000 | 18 000 | 21 000 | 5600 |
| Valve exclusion | Severe valve disorder | Significant MS; any prosthesis | Mod/sev MS; mechanical | Mod/sev MS; mechanical | Requires surgery; mechanical |
| Renal exclusion | CrCl < 30 mL/min | CrCl < 30 mL/min | Cr > 2.5 mg/dL or CrCl < 25 mL/min | CrCl < 30 mL/min | Cr > 2.5 mg/dL or CrCl < 25 mL/min |
| Protocol-specified dose-reduction for renal impairment | None | CrCl 15-50 mL/min: 15 mg daily | ≥2 of following age >80, weight <60 kg, Cr >1.5 mg/dL (2.5 mg twice daily) | ≥1 of the following: CrCl 30-50 mL/min, weight ≤60 kg, concomitant use of potent P-glycoprotein inhibitors (halved dose) | ≥2 of following age >80, weight <60 kg, Cr >1.5 mg/dL (2.5 mg twice daily) |
| CHADS2, mean | 2.1 | 3.5 | 2.1 | 2.8 | 2.0 |
| Triple therapy allowed (OAC+DAPT)28 | Yes | No | No | No | No |
| Stroke outcomes | Noninferior (110 mg), superior (150 mg) | Noninferior | Superior | Noninferior | Superior |
| Bleeding outcomes | Superior (110 mg), noninferior (150 mg) | Noninferior (ICH/fatal vs GI) | Superior | Superior | Not significantly different |
| All-cause mortality reduced | No | No | Yes | Yes (30 mg), | No |
| No (60 mg) |
ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; Cr, creatinine; CrCl, creatinine clearance; DAPT, dual antiplatelet therapy; ENGAGE AF-TIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48; GI, gastrointestinal; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET-AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism.
US Food and Drug Administration–approved dose for stroke prevention in AF is 150 mg twice daily for dabigatran (75 mg twice daily for CrCl 15-30 mL/min), 60 mg daily for edoxaban (30 mg for those with CrCl 15-50 mL/min; edoxaban is contraindicated for stroke prevention in AF for patients with CrCl >95 mL/min), and 60 mg daily for edoxaban.
There are additional considerations beyond trial design, such as dosing frequency (daily for rivaroxaban and edoxaban versus twice-daily for dabigatran and apixaban), GI history (rivaroxaban is associated with increased risk of GI bleeding; dabigatran is associated with dyspepsia),20,27,31 and potential medication interactions (see package labeling). If there is serious concern regarding the potential for specific, significant drug-drug interactions with an NOAC, this may require selection of an alternative therapy because measuring NOAC clinical drug effect in vivo is not available in routine clinical practice. Furthermore, dabigatran is currently the only agent with an approved, targeted reversal agent (although others are in late-stage development).32,33 Patients may also have their own preconceived opinions regarding the anticoagulant of choice, and these could influence treatment as well; the best medication is useless in the patient who will not take it.
Additional considerations
The effectiveness of warfarin for stroke prevention in AF has been shown to fluctuate based on time in therapeutic range (TTR); lower TTR has been associated with increased risk of stroke and bleeding.34,35 Therefore, patients on warfarin with highly variable international normalized ratios may derive significant benefit from transitioning to an NOAC. However, caution should be exercised in and around switching,36 and it should also be noted that several analyses of NOAC trials have demonstrated a consistent benefit for NOACs over warfarin across the spectrum of TTR.24,37,38 Therefore, the decision to switch a patient stable on warfarin to an NOAC should be individualized. Last, in patients who must remain on warfarin despite poor TTR, there are a variety of strategies demonstrated to improve TTR, such as self-monitoring and novel testing.39,40
Many patients will inquire about the use of antiplatelet therapy for stroke prevention in AF, specifically aspirin and/or clopidogrel. Although adding clopidogrel to aspirin can help reduce thromboembolic events,41,42 risk of bleeding is increased, and the net clinical benefit is unclear.43 Furthermore, the Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment trial demonstrated superior stroke prevention without significantly higher rates of bleeding for apixaban compared with aspirin alone.30 Therefore, there are likely very few patients who are appropriate candidates for long-term, antiplatelet-only stroke prevention in AF; they are usually patients who adamantly oppose OAC (or cannot afford it), but whose bleeding risk does not preclude antiplatelet therapy. In current US guidelines, only patients with a CHA2DS2-VASc score of 1 are candidates for aspirin; due to a general lack of evidence, the guidelines allow for any treatment option in this group (none, aspirin, or OAC).7 However, further investigation is needed into the appropriate management of these patients, and it is unlikely that aspirin provides the optimal risk-benefit profile for stroke prevention in these patients.
One of the biggest challenges in the management of patients receiving anticoagulation is the eventual necessity for interruption of therapy in the setting of an invasive procedure or acute illness. Although a full review of perioperative anticoagulation management is beyond the scope of this article, the most common question is whether the patient requires temporary “bridging” anticoagulation with a short-term, parenteral agent.44 Emerging data from observational and clinical trial cohorts now support a more conservative approach to bridging,45,46 consistent with the American College of Chest Physicians guidelines.47 They minimize the use of routine bridging in AF patients and reserve it for those at particularly high risk of acute thromboembolism: patients with mechanical valves or valvular AF and those with a very high risk of AF-related stroke (CHADS2 score ≥3). The guidelines also emphasize the underlying bleeding risk of the patient and the impending surgical procedure: in the low-risk patient undergoing a low risk procedure, interruption may not be necessary at all.
Clinical cases
Patient 1: 74-year-old woman with new-onset AF
A 74-year-old woman presented to clinic in follow-up after being seen in the emergency department for new-onset palpitations. She had a history of hypertension and hypothyroidism. In the emergency department, she was found to be in AF with ventricular rates as high as 130 beats per minute. She noted acute onset of her symptoms, which have persisted over several days, and briefer episodes over the last several months. Her blood pressure was stable, and she was not hypoxic. Physical examination did not reveal any signs of heart failure or acute systemic illness. Her complete blood counts, coagulation profile, and blood chemistries were all normal (estimated creatinine clearance 66 mL/min). Her thyroid function studies were also normal in the setting of replacement therapy. She was administered calcium channel blockade for rate control and received therapeutic-dose subcutaneous enoxaparin, and a synchronized cardioversion was performed following evaluation of the left atrial appendage for thrombus (by transesophageal echocardiogram). At discharge, she was prescribed enoxaparin and warfarin with instructions to follow-up as an outpatient.
Comments about patient 1
This 74-year-old woman presented with a new diagnosis of AF. The prior evaluation did not reveal any immediately reversible cause of her AF, such as uncontrolled hyperthyroidism or acute medical illness. Identifying such factors would be important, as patients with reversible comorbid conditions that are clearly driving new AF and in whom AF has not otherwise been observed may not require long-term treatment of AF once the comorbid disease is controlled. However, in those circumstances, it is prudent to assess risk factors for AF (eg, hypertension, obesity, sleep apnea, etc).
She underwent cardioversion for an AF episode >48 hours in duration, which conferred a small but temporary increased risk of stroke above that of the AF itself (irrespective of whether the cardioversion was via direct current or medically induced). Current guidelines recommend systemic OAC for at least 4 weeks following cardioversion, irrespective of underlying stroke risk, and this could have been initiated before cardioversion.7 Emerging data on the use of NOACs in this setting have been reassuring,48-50 and their use is reasonable in and around cardioversion.
Regarding long-term anticoagulation, she had a CHA2DS2-VASc score of 3 (1 point each for age, sex, and hypertension) and was not extremely high risk for bleeding: she warranted long-term OAC therapy. It was also reasonable to switch her to an NOAC, with a careful discussion that all OAC therapy increases bleeding risk and that is outweighed by stroke-prevention benefits. She did not have any particular features that point toward 1 NOAC over another, although a once-daily regimen may be preferred if she is taking no other twice-daily medicines. The fact that her AF is paroxysmal should have no bearing on recommendations for stroke prevention7; numerous studies have demonstrated very poor temporal correlation between AF episodes and incident stroke events.51,52
Last, the management of her rhythm and rate is beyond the scope of this review; however, given her significant symptoms in AF, careful consideration should be given to a more aggressive rhythm control strategy.
Patient 2: 68-year-old woman with significant coronary artery disease
This patient is a 68-year-old woman with a history of paroxysmal AF, hypertension, diabetes, and coronary artery disease. She is experiencing increasing angina with exertion despite optimal medical therapy and is undergoing cardiac catheterization with planned coronary stenting. Her body mass index is 21 kg/m2, and her renal function is preserved (creatinine clearance 61 mL/min). She is currently treated with rivaroxaban 20 mg daily for stroke prevention as well as aspirin 325 mg for her atherosclerotic disease.
Comments about patient 2
The patient with concomitant AF and atherosclerotic disease is a topic of fervent clinical research. Antiplatelet therapies are the mainstay for prevention of atherothrombotic events in these patients; however, they are also frequently at increased risk of AF-related thromboembolism. This patient’s CHA2DS2-VASc score is 5 (1 point each for sex, age, hypertension, diabetes, and vascular disease). She is already receiving dual therapy (OAC and aspirin) and is about to undergo coronary stenting, which almost invariably requires intensification of antiplatelet therapy to prevent stent thrombosis. Furthermore, she has multiple risk factors for bleeding complication in the setting of catheterization, including her sex, body mass index, and concomitant OAC.
There are several potential approaches to the antithrombotic management of this patient. Her risk should be managed at the time of catheterization; bleeding may be reduced with a radial artery approach (versus femoral) and careful medical management (eg, dose and selection of glycoprotein IIb/IIIa and/or P2Y12 inhibitors [ie, clopidogrel vs prasugrel or ticagrelor]); stent selection (bare metal vs drug eluting vs newer-generation drug eluting) could influence the intensity and duration of concomitant antiplatelet therapy; and her aspirin dose should be reduced (81 mg).53 There is mounting evidence that aspirin may not be needed at all in this scenario, and it does increase the risk of bleeding19,54-56 ; however, any discussion around aspirin discontinuation should involve the cardiac interventionalist. In addition, a proton pump inhibitor may be reasonable to reduce her risk of GI bleeding.57 These measures will ensure her exposure to the lowest incremental bleeding risk for the shortest period of time. Last, some consensus documents suggest that in the setting of triple therapy (OAC+DAPT), a lower-dose NOAC should be used.53 This recommendation is in part due to clinical trials of standard-dose NOACs in patients with acute coronary syndromes, which demonstrated prohibitive increases in bleeding (albeit a different clinical population).58,59 Although this may be reasonable, these lower doses have not been rigorously evaluated in this setting (clinical trials are ongoing).60,61
Patient 3: 82-year-old man with AF and extreme risk
This 82-year-old man has a history of persistent AF, hypertension, and a prior transient ischemic attack (TIA). His estimated creatinine clearance is 53 mL/min. Because of a history of hemorrhoidal bleeding and patient preference, he was previously treated with aspirin alone until 2 years ago when he presented with an ICH. This ICH was found to be related to a cerebrovascular arteriovenous malformation that was coiled. Currently, he is not receiving any treatment of stroke prevention and presents for evaluation.
Comments about patient 3
Intracranial bleeding is the most feared adverse consequence of OAC therapy, and patients with prior ICH in the absence of OAC represent a particularly high-risk cohort. Nevertheless, not all ICH is the same, and consultation with the patient’s neurologist can be very informative regarding an individual patient’s risk of recurrent ICH. Emerging data suggest prior ICH should not be an absolute contraindication to OAC,62 and this patient’s risk factor for ICH appears to have been controlled (it was coiled). Furthermore, his risk of stroke is very high: the CHA2DS2-VASc score of 5 (2 points each for age and prior TIA, 1 point for hypertension).
Any decision regarding stroke prevention strategy should involve close coordination with his neurology team and a thorough discussion with the patient about the risks and benefits of various strategies. Approaches may include no stroke prevention therapy, if the risk and consequences of recurrent ICH are worse than a thromboembolic event. If OAC is used for any period in this patient, an NOAC may be preferred due to a lower risk of ICH. However, this is a patient that could be counseled about the availability of nonpharmacological stroke prevention strategies (ie, left atrial appendage occlusion), if his operative risk is not prohibitive.63-65 It should be recognized, though, that left atrial appendage procedures (surgical or catheter-based) frequently require at least short-term systemic anticoagulation in the perioperative period, and NOACs were not tested in the device trials left atrial appendage occlusion.
Conclusions
Management of anticoagulation in AF involves a careful balance between risk of AF-related thromboembolism and risk of OAC-related bleeding. Generally, patients with valvular AF and those with nonvalvular AF plus a CHA2DS2-VASc score of ≥2 should receive OAC to prevent stroke. In eligible patients, the cumulative data support a preference for NOACs over warfarin; however, not all patients are candidates to receive an NOAC. Among patients without a contraindication to NOAC therapy, selection among the NOAC agents is primarily left to physician and patient discretion. Important patient groups remain underrepresented in major clinical trials, including patients with advanced age, morbid obesity, severe chronic kidney disease, and those with a CHA2DS2-VASc score of 1. Management of such patients can be particularly challenging, and additional research is needed. Patient engagement in decisions regarding OAC is vital to optimal implementation of strategies for stroke prevention in AF.
Authorship
Contribution: B.A.S. wrote the manuscript.
Conflict-of-interest disclosure: B.A.S. receives receive research support from Janssen Pharmaceuticals and received consultant support from Bristol-Myers Squibb–Pfizer.
Correspondence: Benjamin A. Steinberg, University of Utah Health Sciences Center, 30 North 1900 East, Room 4A100, Salt Lake City, UT 84132; e-mail: benjamin.steinberg@hsc.utah.edu.