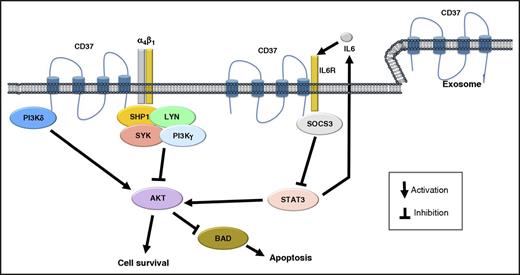
Schematic representation of the biologic role of CD37 in B cells. CD37 has both prosurvival and proapoptotic capacities. It can mediate the clustering of α4β1 integrins, which is followed by activation of PI3K/AKT signaling and cell survival. However, CD37 can also create a complex with SHP1, LYN, SYK, and PI3Kγ, leading to AKT inactivation, and recruit SOCS3, limiting the IL6/STAT3 loop.
Schematic representation of the biologic role of CD37 in B cells. CD37 has both prosurvival and proapoptotic capacities. It can mediate the clustering of α4β1 integrins, which is followed by activation of PI3K/AKT signaling and cell survival. However, CD37 can also create a complex with SHP1, LYN, SYK, and PI3Kγ, leading to AKT inactivation, and recruit SOCS3, limiting the IL6/STAT3 loop.
CD37 is a heavily glycosylated protein that belongs to the family of the tetraspanins, a group of transmembrane proteins involved in cell membrane organization and co-signaling.2,3 It is expressed almost exclusively in cells of the immune system, especially in mature B cells, but also, at lower levels, in T cells, macrophages/monocytes, granulocytes, and dendritic cells.2 CD37 is present on the cell surface, but also at the level of endosomes and exosomes, thus contributing to intracellular trafficking and immunomodulation. In physiological conditions, CD37 interacts with a variety of molecules, including PI3Kγ (PIK3CG), PI3Kδ (PIK3CD), SYK, LYN, CD19, CD22, SHP1, IL6R, SOCS3, CD53, CD81, CD82, major histocompatibility complex (MHC) class II, and different integrins such as ITGB1 (integrin β1), ITGB2 (integrin β2), ITGB3 (integrin β3), and ITGB7 (integrin β7) (as obtained on the STRING database at http://string-db.org2,-4 ). The biologic function of CD37 is not yet fully elucidated, but it may be involved both in prosurvival and proapoptotic signaling (see figure). Mice lacking CD37 present a reduced number of antibody secreting cells and of memory B cells produced in the context of a T-cell-dependent response,2 and this appears to be a result of the lack of a CD37-mediated clustering of α4β1 integrins (VLA-4) on germinal center B cells, with reduced downstream activation of PI3K/AKT signaling and cell survival.2 However, older mice lacking CD37 develop germinal center-derived B-cell lymphomas as a result of the loss of a CD37-mediated negative feedback on the IL6/STAT3 axis.4 Moreover, CD37 can also signal via a complex, including SHP1, LYN, SYK, and PI3Kγ, resulting in AKT inactivation and cell death.3
Mirroring the expression pattern seen in normal lymphocytes, CD37 is highly expressed across all mature B-cell lymphoid neoplasms, including chronic lymphocytic leukemia, whereas it is low/absent in acute lymphoblastic leukemias, Hodgkin lymphoma, and multiple myeloma.5,-7 T-cell lymphomas can also express CD37.5,6 A wide range of CD37 positivity (from more than 90% to 40% of cases) has been reported in DLBCL,1,5,-7 which could be partially a result of the staining approach (flow cytometry vs immunohistochemistry), the antibodies, or the laboratory protocols used. Indeed, in the paper by Xu-Monette et al, many cases negative at protein level do still express high levels of CD37 RNA, and it is likely that antibodies recognizing different epitopes of CD37, which, as already mentioned, undergoes heavy posttranslational modification (eg, glycosylation), might give different results.
On the basis of an expression pattern largely overlapping CD20, CD37 was one of the first proteins explored as therapeutic target in non-Hodgkin lymphomas in the late 1980s with the 131I MB-1 radioimmunoconjugate. A renewed interest in this target has supported the development of new therapeutic agents targeting CD37, with naked monoclonal antibodies (BI836826), small modular immunopharmaceuticals (the single-chain, homodimeric therapeutic protein Otlertuzumab/TRU-016), antibody drug conjugates (IMGN529 and AGS67E, conjugated to the maytansinoid DM1 or to the monomethyl auristatin E, respectively), and novel radioimmunoconjugates (177Lu Betalutin) currently in clinical investigation.6,,,-10 Clinical activity has been observed in chronic lymphocytic leukemia, indolent and DLBCL, and some of these agents have now moved to combination studies with rituximab, chemotherapy, or targeted agents (see the following studies at ClinicalTrials.gov: NCT00614042, NCT01188681, NCT01317901, NCT01534715 NCT01403948, NCT01403948, NCT01644253, NCT01796171, NCT02175433, NCT02538614, NCT02564744, NCT02624492, NCT02658968, NCT02759016). CD37 staining is not routinely performed in the clinical practice. There is preclinical evidence suggesting CD37 expression levels do not correlate with therapeutic benefit5,7 ; however, this will need validation in clinical studies.
Xu-Monette et al have provided interesting new insights on the biologic role of CD37 in human DLBCL in relation to its expression across a very large number of homogeneously treated patients. Further studies should be performed to properly address the relationship between CD37 and sensitivity to the anti-CD20 monoclonal antibody rituximab and to harmonize staining protocols before including CD37 staining as a novel prognostic marker in the diagnostic workup for patients with DLBCL.
Conflict-of-interest disclosure: The authors have received institutional research grants from Immunogen (F.B., A.S.) and Nordic Nanovector (F.B.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal