Key Points
Activation of mTORC1 in MKs and platelets contributes to aging-related venous thrombosis.
ROS production with aging activates mTORC1 to elevate MPV and platelet activation and promote venous thrombosis.
Abstract
Aging is associated with an increased incidence of venous thromboembolism (VTE), resulting in significant morbidity and mortality in the elderly. Platelet hyperactivation is linked to aging-related VTE. However, the mechanisms through which aging enhances platelet activation and susceptibility to VTE are poorly understood. In this study, we demonstrated that mechanistic target of rapamycin complex 1 (mTORC1) signaling is essential for aging-related platelet hyperactivation and VTE. mTORC1 was hyperactivated in platelets and megakaryocytes (MKs) from aged mice, accompanied by elevated mean platelet volume (MPV) and platelet activation. Inhibition of mTORC1 with rapamycin led to a significant reduction in susceptibility to experimental deep vein thrombosis (DVT) in aged mice (P < .01). To ascertain the specific role of platelet mTORC1 activation in DVT, we generated mice with conditional ablation of the mTORC1-specific component gene Raptor in MKs and platelets (Raptor knockout). These mice developed markedly smaller and lighter thrombi, compared with wild-type littermates (P < .01) in experimental DVT. Mechanistically, increased reactive oxygen species (ROS) production with aging induced activation of mTORC1 in MKs and platelets, which, in turn, enhanced bone marrow MK size, MPV, and platelet activation to promote aging-related VTE. ROS scavenger administration induced a significant decrease (P < .05) in MK size, MPV, and platelet activation in aged mice. Our findings collectively demonstrate that mTORC1 contributes to enhanced venous thrombotic susceptibility in aged mice via elevation of platelet size and activation.
Introduction
Venous thrombosis (or venous thromboembolism [VTE]), a disease that includes both deep vein thrombosis (DVT) and pulmonary embolism, is the third most common cardiovascular disorder among the elderly, which results in significant morbidity and mortality in the elderly.1,2 This condition is rare in young individuals (<1 per 10 000 per year) but increases to ∼1% per year in the elderly population.3 Despite the well-established clinical association between aging and VTE,4,5 the mechanisms by which aging increases susceptibility to VTE remain largely unknown.
Platelets are anucleated cells derived from megakaryocytes (MKs). Platelet activation is a link in the pathophysiology of diseases prone to thrombosis and is viewed as a therapeutic target in thromboembolism.6-9 Platelet size, measured as mean platelet volume (MPV), is considered a marker of its function and positively associated with indicators of platelet activity, including aggregation and production of thrombogenic factors, such as thromboxane A2, von Willebrand factor (VWF), platelet factor 4 (PF4), β-thromboglobulin, and P-selectin.10-12 A number of population studies have shown that MPV and platelet activation increase with age,13 implying an essential role of these factors in aging-related VTE. Increased MPV is viewed as a predictor of unprovoked VTE.14,15 However, the signaling pathways underlying the production of large-sized platelets in bone marrow and platelet hyperactivation during aging are yet to be clarified.
The mechanistic target of rapamycin complex 1 (mTORC1) consisting of mTOR, Raptor, and mLST8 is sensitive to rapamycin and thought to control autonomous cell growth in response to nutrient availability and growth factors.16-18 Recent studies have established mTORC1 as a key modulator of aging and age-related diseases.19,20 Inhibition of the mTORC1 pathway has been shown to extend the life span of model organisms and confer protection against some age-related pathologies.21,22 Although mTORC1 clearly plays critical roles in the proliferation of MK progenitors, late-stage differentiation of MKs, and platelet activation,23-26 its role in venous thrombosis remains to be defined. Studies on patients receiving rapalogs after liver or kidney transplantation have reported inhibitory, stimulatory, or no effects of rapamycin on arterial thrombosis.27-32 Individual variations, treatment periods, and targeting of multiple cells within the vascular system, including monocytes, endothelial cells, neutrophils, and platelets, by rapamycin may contribute to these conflicting results. The specific roles of platelet mTORC1 in age-related VTE and underlying mechanisms have not yet been established.
In the present study, we demonstrated an increase in mTORC1 activity in MKs and platelets in aging mice. Inhibition of mTORC1 with rapamycin or MK- and platelet-specific deletion of Raptor (the mTORC1 component) reduced the age-related increase in MK size, MPV, platelet activation, and susceptibility to DVT in mice. Our findings provide mechanistic insights into aging-related thrombosis and support the utility of strategies to lower MK and platelet mTORC1 activity to abrogate enhanced MPV and activation of platelets from aged mice as a potentially effective therapeutic approach.
Methods
Mice and treatments
Female C57BL/6 mice were purchased from the Laboratory Animal Centre of the Southern Medical University (Guangzhou, China). According to the accepted principles for experiments on the biology of aging in mice, animals at 4 and 16 months of age were used. Mice were administered rapamycin (LC Laboratories, Woburn, MA) (1.5 mg/kg per d) intragastrically for 2 months (n = 10 per group) to inhibit mTORC1 or exposed to the reactive oxygen species (ROS) scavenger N-acetyl-l-cysteine (NAC) (2 mg/mL) or dl-buthionine-(S,R)-sulfoximine (BSO) (15 mM) (Sigma-Aldrich Corporation, St. Louis, MO) in drinking water for 2 months (n = 10). PF4-Cre and Raptorflox/flox mice (Jax #008535 and #013188, respectively) were purchased from the Jackson Laboratory (Bar Harbor, ME). PF4-Cre mice were crossed with Raptorflox/flox mice to generate mice lacking Raptor in platelets and MKs. Genotyping was performed via polymerase chain reaction of tail DNA and Raptor deficiency in platelets confirmed via western blot. The Animal Care and Use Committee of the Southern Medical University approved this research protocol.
Experimental DVT
An inferior vena cava (IVC) ligation model was used as described previously.7 The IVC and all visible side branches (usually 2 or 3) were ligated with nonreactive 6-0 silk sutures. Two days later, the IVC and the associated thrombus of each group were removed, weighed, and measured for thrombus length.
Statistical analyses
All results are presented as the mean ± standard error of the mean (SEM). Analysis was performed with SPSS 19.0 statistical software (IBM-SPSS Inc., Chicago, IL). Parametrical results were compared using the unpaired Student t test. Nonparametrical data without normal Gaussian distribution were compared using the Mann-Whitney U test. Asterisks indicate statistically significant differences (*P < .05, **P < .01, and ***P < .001), as compared with respective controls.
Results
mTORC1 activation in MKs and platelets is enhanced in aged mice
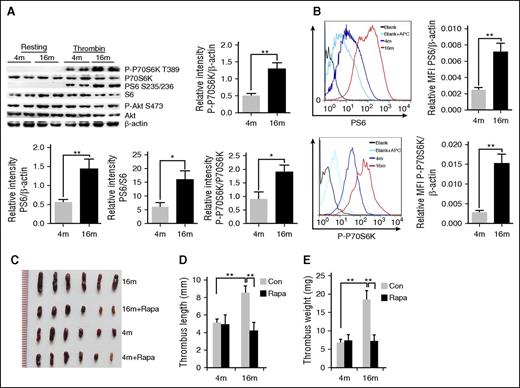
To investigate the role of platelet mTORC1 in age-related venous thrombosis, we initially examined the relationship between age and platelet and MK mTORC1 activity in mice. Thrombin-induced platelet mTORC1 activation (phosphorylation [P] of P70S6K at threonine 389 and S6 at serine 235/236) in aged (16-month-old) mice was significantly enhanced, as compared with that in young (4-month-old) mice (Figure 1A; supplemental Figure 1A, available on the Blood Web site). In contrast, phosphorylation of the mTORC2 substrate, Akt (S473, also known as protein kinase B), remained unaltered with age, suggesting specific mTORC1 activation in platelets. Fluorescence-activated cell sorting (FACS) analysis of bone marrow cells revealed that P-P70S6K and P-S6 were also increased in MKs of aged mice (Figure 1B; supplemental Figure 1B). Interestingly, total protein expression in the same numbers of platelets or MKs was also enhanced in aged mice, implicating that increased platelet and MK size might occur in aged mice (supplemental Figure 1C). These findings collectively suggest that mTORC1 activity in MKs and platelets increases with age in mice.
Enhanced mTORC1 activity in MKs and platelets is associated with age-related increased susceptibility to VTE in mice. (A) Platelets isolated from 4- and 16-month-old mice were stimulated with thrombin (0.2 U/mL) and subjected to western blot analysis for mTORC1 (P-S6, P-P70S6K) and mTORC2 (P-Akt) activities (n = 16). Relative values are provided for immunoreactivity for P-S6, P-P70S6K related to the S6, P70S6K and β-actin signals. Values represent means ± SEM; **P < .01. (B) FACS analysis of MKs from 4- or 16-month-old mice for mTORC1 activity (P-S6, P-P70S6K) (n = 4). Mean fluorescent intensity (MFI) values represent means ± SEM; **P < .01. Images (C), lengths (D), and weights (E) of thrombi that developed in the IVC 48 hours after ligation were measured in 4- or 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months (n = 6). Values represent means ± standard error (SE). **P < .01.
Enhanced mTORC1 activity in MKs and platelets is associated with age-related increased susceptibility to VTE in mice. (A) Platelets isolated from 4- and 16-month-old mice were stimulated with thrombin (0.2 U/mL) and subjected to western blot analysis for mTORC1 (P-S6, P-P70S6K) and mTORC2 (P-Akt) activities (n = 16). Relative values are provided for immunoreactivity for P-S6, P-P70S6K related to the S6, P70S6K and β-actin signals. Values represent means ± SEM; **P < .01. (B) FACS analysis of MKs from 4- or 16-month-old mice for mTORC1 activity (P-S6, P-P70S6K) (n = 4). Mean fluorescent intensity (MFI) values represent means ± SEM; **P < .01. Images (C), lengths (D), and weights (E) of thrombi that developed in the IVC 48 hours after ligation were measured in 4- or 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months (n = 6). Values represent means ± standard error (SE). **P < .01.
Rapamycin protects aged mice from accelerated DVT
Next, we examined the effects of rapamycin administration on experimental DVT in young (4-month-old) and aged (16-month-old) C57BL/6 mice. Consistent with previous results,33,34 aged mice developed significantly larger and heavier IVC thrombi and more platelets in the thrombi, as compared with young mice (P < .01) (Figure 1C-E; supplemental Figure 2A). Interestingly, 4-month-old mice with and without 2 months of rapamycin (1.5 mg/kg per day) treatment developed thrombi of similar lengths and weights (P < .5) (Figure 1C-D), implying that rapamycin does not affect susceptibility to VTE in young mice. However, in 16-month-old mice, rapamycin protected against the age-dependent effect, as evident from the significantly smaller thrombi in treated animals relative to age-matched vehicles (P < .01) (Figure 1C-E). Similar results were found in a ferric chloride–induced carotid artery thrombosis model (supplemental Figure 2B). Our findings suggest that inhibition of mTORC1 by rapamycin reduces the susceptibility of aged mice to experimental thrombosis.
Deletion of mTORC1 in MKs and platelets reduces susceptibility to DVT in mice
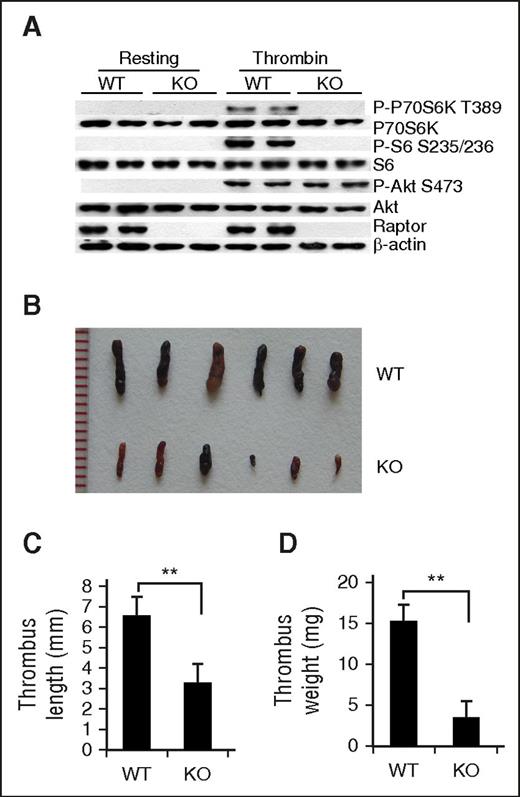
Whole-body rapamycin treatment may affect various types of thrombosis-related cells within the vascular system, such as monocytes, endothelial cells, neutrophils, and platelets.35 To identify the specific role(s) of platelet mTORC1 activation in VTE, we generated mice with conditional ablation of the mTORC1-specific component, Raptor, in MKs and platelets using a Cre recombinase expression cassette under control of the Pf4 promoter (Raptor knockout [KO]). Raptor KO mice were born at the expected Mendelian frequency, and recombination and deletion of Raptor alleles only occurred in MKs and platelets, as demonstrated with allele-specific polymerase chain reaction (supplemental Figure 3). Western blot data revealed inhibition of P-P70S6K and P-S6 in thrombin-stimulated platelets of Raptor KO mice (Figure 2A), indicating that mTORC1 is inactivated upon Raptor disruption.
Deletion of mTORC1 in MKs and platelets reduces susceptibility to venous thrombosis in mice. (A) Western blot analysis of activated or resting platelets from platelet-specific Raptor-deletion (KO) or wild-type (WT) mice for Raptor, P-S6, and P-P70S6K expression. A typical result of platelets from 3 different mice is shown. Images (B), lengths (C), and weights (D) of thrombi that developed in the IVC 48 hours after ligation were measured in 12-month-old WT and Raptor KO mice (n = 6). Values represent mean ± SE. **P < .01.
Deletion of mTORC1 in MKs and platelets reduces susceptibility to venous thrombosis in mice. (A) Western blot analysis of activated or resting platelets from platelet-specific Raptor-deletion (KO) or wild-type (WT) mice for Raptor, P-S6, and P-P70S6K expression. A typical result of platelets from 3 different mice is shown. Images (B), lengths (C), and weights (D) of thrombi that developed in the IVC 48 hours after ligation were measured in 12-month-old WT and Raptor KO mice (n = 6). Values represent mean ± SE. **P < .01.
No significant differences in body weight or length were observed between Raptor-deficient mice and littermate controls at all ages. There was also no obvious hemorrhaging or intestinal bleeding observed in Raptor-deficient mice (supplemental Figure 4). Strikingly, both old and young Raptor KO mice subjected to IVC ligation developed significantly smaller and lighter thrombi, as compared with their WT littermates (P < .01) (Figure 2B-D). Rapamycin treatment did not further reduce the thrombi in Raptor-deletion mice (supplemental Figure 5), clearly implying that platelet Raptor deletion provides protection against stasis-induced VTE development in mice.
Inhibition of mTORC1 decreases age-related elevation of MPV and platelet activation in mice
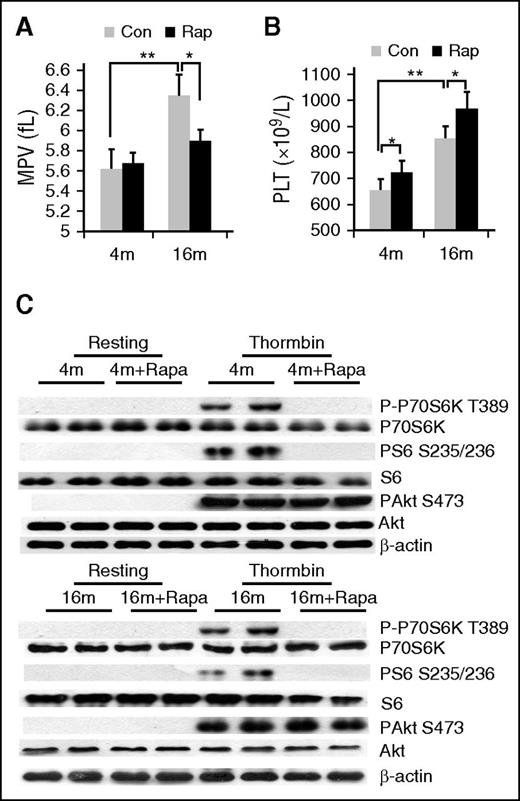
To further ascertain the mechanism through which platelet mTORC1 promotes venous thrombosis, we examined the effects of rapamycin on MPV, an indicator of platelet size and marker of platelet function. Both MPV and platelet count were markedly increased in 16-month-old mice, compared with 4-month-old mice (P < .01) (Figure 3A-B). After rapamycin treatment of 2 months, MPV of aged, but not young, mice was significantly reduced (P < .05) (Figure 3A). In contrast, platelet counts were slightly enhanced by rapamycin both in young and aged mice (Figure 3B). Western blot analysis confirmed inhibition of platelet mTORC1 (P-S6), but not mTORC2 (P-Akt) in rapamycin-treated mice (Figure 3C). These findings indicate that specific inhibition of mTORC1 via long-term administration of rapamycin can suppress MPV in mice.
Rapamycin suppresses age-associated elevation of MPV in mice. (A) Complete blood count analysis of 4- or 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months. (A) MPV and (B) platelet counts are shown (n = 10). Values represent means ± SEM; *P < .05; **P < .01. (C) Western blot analysis of platelets from mice described in panel A for mTORC1 (P-S6, P-P70S6K) activity.
Rapamycin suppresses age-associated elevation of MPV in mice. (A) Complete blood count analysis of 4- or 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months. (A) MPV and (B) platelet counts are shown (n = 10). Values represent means ± SEM; *P < .05; **P < .01. (C) Western blot analysis of platelets from mice described in panel A for mTORC1 (P-S6, P-P70S6K) activity.
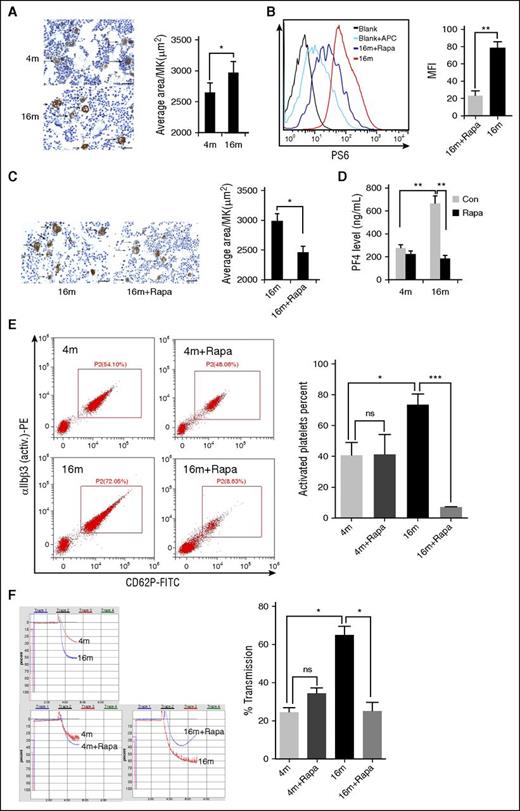
The size of platelets (MPV) is determined by the megakaryopoiesis process in bone marrow.6,36 Accordingly, we examined the effects of rapamycin on MKs. Interestingly, the average size of bone marrow MKs in 16-month-old mice was ∼10% greater than in 4-month-old mice (Figure 4A), whereas the MK number did not differ significantly between the age groups (supplemental Figure 6). Increased MPV and MK size may contribute to the enhanced total protein expression in the same numbers of platelets and MKs in aged mice. Two months of rapamycin treatment led to inhibition of MK mTORC1 (Figure 4B) and reduction in the average size of MKs in aged mice (Figure 4C). Clearly, long-term administration of rapamycin can reduce the age-related elevation of bone marrow MK size.
Inhibition of mTORC1 suppresses age-associated elevation of MK size and platelet activation in mice. (A) VWF immunohistochemistry (IHC) staining of femur bone sections from 4- or 16-month-old mice. Bar represents 100 μm. Quantification of MK sizes (average area per MK) is illustrated (n = 10). Values represent means ± SEM; *P < .05. (B) FACS analysis of MKs from 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months for mTORC1 activity (P-S6) (n = 10). MFI values are presented as means ± SEM; **P < .01. (C) VWF IHC staining of femur bone sections from 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months (n = 10). Bar represents 100 μm. Quantification of average area per MK is illustrated. *P < .05. (D) PF4 enzyme-linked immunosorbent assay (ELISA) analysis of plasma from 4- or 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months (n = 10). Values represent means ± SEM; **P < .01. (E) FACS analysis of platelets incubated with 0.2 U/mL thrombin for 15 minutes at room temperature from mice described in panel D for platelet integrin αIIbβ3 activation and quantification illustrated (n = 10). Values represent means ± SEM; *P < .05; ***P < .001. (F) Adenosine 5′-diphosphate–induced (20 μM) platelet aggregation in platelet-rich plasma from mice described in panel D (n = 10). Values represent means ± SEM; *P < .05.
Inhibition of mTORC1 suppresses age-associated elevation of MK size and platelet activation in mice. (A) VWF immunohistochemistry (IHC) staining of femur bone sections from 4- or 16-month-old mice. Bar represents 100 μm. Quantification of MK sizes (average area per MK) is illustrated (n = 10). Values represent means ± SEM; *P < .05. (B) FACS analysis of MKs from 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months for mTORC1 activity (P-S6) (n = 10). MFI values are presented as means ± SEM; **P < .01. (C) VWF IHC staining of femur bone sections from 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months (n = 10). Bar represents 100 μm. Quantification of average area per MK is illustrated. *P < .05. (D) PF4 enzyme-linked immunosorbent assay (ELISA) analysis of plasma from 4- or 16-month-old mice treated with rapamycin (1.5 mg/kg per day) for 2 months (n = 10). Values represent means ± SEM; **P < .01. (E) FACS analysis of platelets incubated with 0.2 U/mL thrombin for 15 minutes at room temperature from mice described in panel D for platelet integrin αIIbβ3 activation and quantification illustrated (n = 10). Values represent means ± SEM; *P < .05; ***P < .001. (F) Adenosine 5′-diphosphate–induced (20 μM) platelet aggregation in platelet-rich plasma from mice described in panel D (n = 10). Values represent means ± SEM; *P < .05.
To further determine whether increased platelet mTORC1 activity and MPV enhance platelet activation and function in aged mice, we examined the production of thrombogenic factors, including PF4 and P-selectin, and activation of αIIbβ3 and platelet aggregation from 4- and 16-month-old mice with or without 2 months of rapamycin treatment. As expected, we observed an age-dependent increase in the production of PF4 and P-selectin, αIIbβ3 activation, and platelet aggregation. Rapamycin treatment suppressed PF4 production, αIIbβ3 activation, and platelet aggregation in aged mice (Figures 4D-F; supplemental Figure 12). Surprisingly, the plasma P-selectin level in both young and old mice was enhanced in the presence of rapamycin (supplemental Figure 7), implying that inhibition of mTORC1 stimulates P-selectin secretion into plasma by endothelial cells.
Collectively, these findings demonstrate that inhibition of mTORC1 prevents age-related elevation of MK size and MPV and platelet activation in mice.
Deletion of mTORC1 reduces MPV and MK size and represses platelet activation in mice
To investigate the consequences of mTORC1 inhibition specifically in MKs and platelets, we compared MKs and platelet formation and activation in Raptor-deletion and control mice. MPV, but not platelets, of 4-, 12-, and 16-month-old Raptor KO mice was significantly reduced, as compared with littermate controls (P < .05 or P < .01) (Figure 5A-B). Although the MK numbers in Raptor KO mice did not differ significantly from that in WT mice (Figure 5C-D), the average size of bone marrow MKs was ∼13% smaller than that of WT mice (Figure 5E). Although there were also no apparent ultrastructural differences between Raptor-null and WT MKs, Raptor-deficient MKs exhibited a reduced proplatelet and ploidy formation and decreased sensitivity to thrombopoietin (Figure 6A-B; supplemental Figure 8). Furthermore, although WT and Raptor-null platelets have comparable levels of cell surface receptors for platelet activation (supplemental Figure 9), the activation of αIIbβ3, platelet aggregation, spreading, and production of PF4 were reduced in Raptor KO mice (P < .05 or P < .01) (Figure 6C-F; supplemental Figure 12). Similarly, fibrin and clot formation were reduced in Raptor-deletion mice (Figure 6G-H), and the in vivo tBT in Raptor KO mice was much higher than that of WT mice (Figure 6I). Raptor deletion also enhanced autophagic flux, as manifested by increased levels of cleaved light chain 3b and autophagosome number in MKs, indicating that mTORC1 inhibits autophagy by platelets and MKs, as in other cell types (supplemental Figure 10).
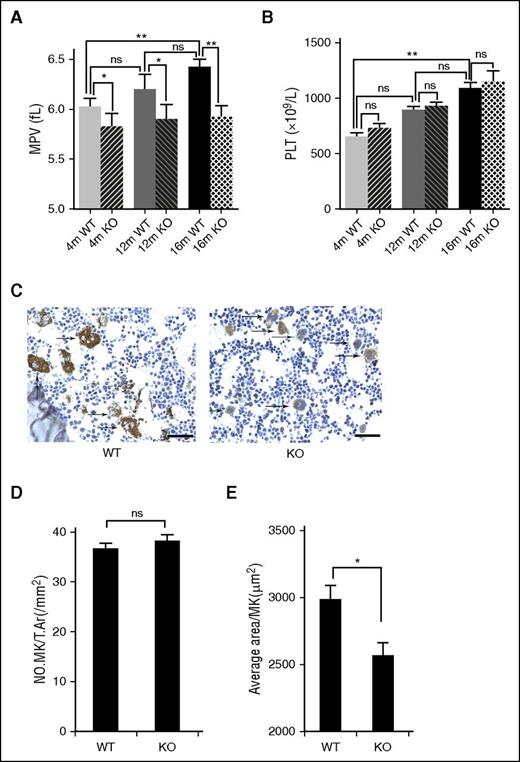
Deletion of mTORC1 suppresses age-associated elevation of MPV and MK size in mice. (A) Complete blood count analysis of 4-, 12-, and 16-month-old WT and Raptor KO mice. MPV (A) and platelet (PLT) (B) counts are shown (n = 8). Values represent means ± SEM; *P < .05; **P < .01. (C) VWF IHC staining of femur bone sections from 4- and 12-month-old WT and Raptor KO mice. Bar represents 100 μm. Quantification of number, no. MK/T.Ar(/mm2), (D) and MK size (E). *P < .05.
Deletion of mTORC1 suppresses age-associated elevation of MPV and MK size in mice. (A) Complete blood count analysis of 4-, 12-, and 16-month-old WT and Raptor KO mice. MPV (A) and platelet (PLT) (B) counts are shown (n = 8). Values represent means ± SEM; *P < .05; **P < .01. (C) VWF IHC staining of femur bone sections from 4- and 12-month-old WT and Raptor KO mice. Bar represents 100 μm. Quantification of number, no. MK/T.Ar(/mm2), (D) and MK size (E). *P < .05.
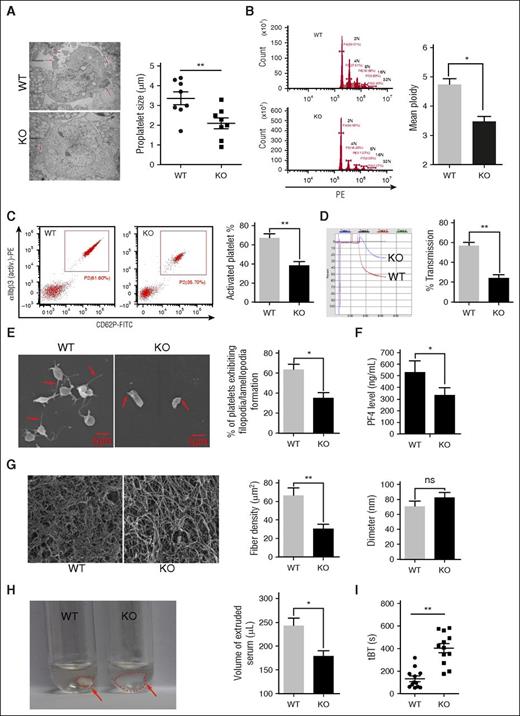
Deletion of mTORC1 suppresses MK differentiation and platelet function. (A) Ultrastructure and proplatelet formation of MKs from 12-month-old Raptor KO and WT mice (n = 8). Values represent means ± SEM; **P < .01. (B) Ploidy formation of 12-month-old WT and Raptor KO mice (n = 8). Values represent means ± SEM; *P < .05. (C) FACS analysis of platelets incubated with 0.2 U/mL thrombin for 15 minutes at room temperature from mice described in panel A for platelet integrin αIIbβ3 activation and quantification illustrated (n = 8). Values represent means ± SEM; **P < .01. Adenosine 5′-diphosphate–induced (20 μM) platelet aggregation in PRP (D) and thrombin-induced (0.2 U/mL) platelet spreading analysis (E) from mice described in panel A (n = 8). Values represent means ± SEM; **P < .01; *P < .05. (F) PF4 ELISA analysis of plasma from 12-month-old WT and Raptor KO mice. Values represent means ± SEM; *P < .05. WT and Raptor KO mice were subjected to fibrin formation (G) and clot formation (H) assays (n = 5). Values represent means ± SEM; **P < .01; *P < .05. (I) Tail bleeding time (tBT) analysis of WT and Raptor KO mice (n = 10). Values represent means ± SEM; **P < .01.
Deletion of mTORC1 suppresses MK differentiation and platelet function. (A) Ultrastructure and proplatelet formation of MKs from 12-month-old Raptor KO and WT mice (n = 8). Values represent means ± SEM; **P < .01. (B) Ploidy formation of 12-month-old WT and Raptor KO mice (n = 8). Values represent means ± SEM; *P < .05. (C) FACS analysis of platelets incubated with 0.2 U/mL thrombin for 15 minutes at room temperature from mice described in panel A for platelet integrin αIIbβ3 activation and quantification illustrated (n = 8). Values represent means ± SEM; **P < .01. Adenosine 5′-diphosphate–induced (20 μM) platelet aggregation in PRP (D) and thrombin-induced (0.2 U/mL) platelet spreading analysis (E) from mice described in panel A (n = 8). Values represent means ± SEM; **P < .01; *P < .05. (F) PF4 ELISA analysis of plasma from 12-month-old WT and Raptor KO mice. Values represent means ± SEM; *P < .05. WT and Raptor KO mice were subjected to fibrin formation (G) and clot formation (H) assays (n = 5). Values represent means ± SEM; **P < .01; *P < .05. (I) Tail bleeding time (tBT) analysis of WT and Raptor KO mice (n = 10). Values represent means ± SEM; **P < .01.
These results suggest that mTORC1 is essential for megakaryopoiesis, as well as platelet formation and activation. Deletion of mTORC1 leads to the generation of small-sized MKs and platelets, resulting in defects in production of thrombogenic factors and platelet activation.
ROS are involved in mTORC1-dependent elevation of MPV and platelet activation during aging
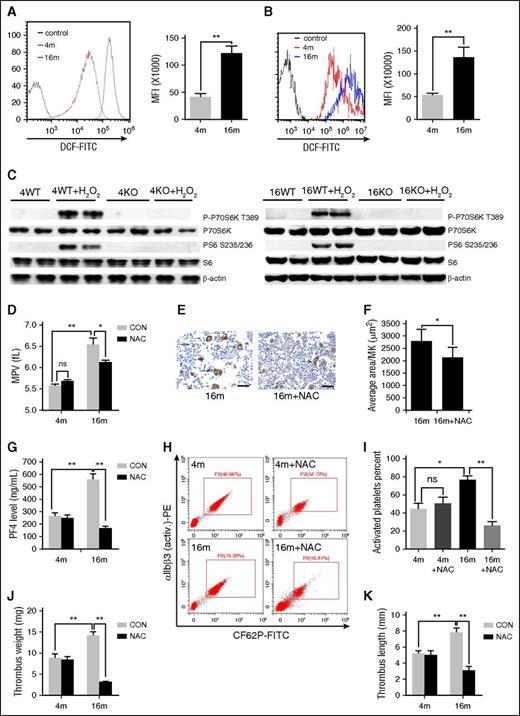
Next, we examined the potential mechanisms leading to aging-dependent activation of mTORC1 in MKs and platelets. Previous studies have established an important role of ROS, such as hydrogen peroxide (H2O2), in aging-related platelet hyperactivation and thrombosis.34,37 Our group reported activation of mTORC1 by low concentrations of H2O2 in a variety of cells.38 As expected, higher levels of ROS were observed both in platelets and in MKs from 16-month-old mice (P < .01 vs 4-month-old mice) (Figure 7A-B). H2O2 induced mTORC1 activation in platelets from 4- and 16-month-old mice, but not in Raptor-null platelets, in vitro (Figure 7C). To establish the correlations between ROS with mTORC1 activation and platelet formation in vivo, aged mice were administered the ROS scavenger NAC. Notably, platelet P-S6 levels (supplemental Figure 11), MPV (Figure 7D), average bone marrow MK size (Figure 7E-F), PF4 level (Figure 7G), αIIbβ3 activation (Figure 7H-I; supplemental Figure 12), and venous thrombus size of 16-month-old mice were significantly reduced after NAC treatment (Figure 7J-K). NAC could not further decrease MPV in Raptor KO mice (supplemental Figure 11). On the contrary, administration of BSO, a glutathione-depleting agent, for 2 months produced high levels of ROS and enhanced platelet count and MPV in young mice (supplemental Figure 13). However, BSO could not enhance MPV and MK size in Raptor-deletion mice (supplemental Figure 13). Based on these findings, we propose that ROS contribute to mTORC1-dependent elevation of MPV and platelet activation during aging.
H2 O2 contributes to mTORC1-dependent platelet activation during aging. FACS analysis of platelets (A) and MKs (B) from 4- or 16-month-old mice for intracellular H2O2 levels (n = 10). MFI values represent means ± SEM; **P < .01. (C) Western blot analysis of mouse platelets from 4- and 16-month-old WT or Raptor KO mice stimulated with 50 μM H2O2 for 30 minutes for mTORC1 activity. (D) Complete blood count analysis of 4- and 16-month-old mice administered NAC for 2 months (n = 10). MPV and platelets are shown. Values represent means ± SEM; *P < .05; **P < .01. (E) VWF IHC staining of femur bone sections from mice described in panel D. (F) Quantification of MK size is illustrated. Values represent means ± SEM; *P < .05. (G) PF4 ELISA analysis of plasma from mice described in panel D. Values represent means ± SEM; **P < .01. FACS analysis (H) of platelets incubated with 0.2 U/mL thrombin for 15 minutes at room temperature from mice described in panel D for integrin αIIbβ3 activation (n = 10) and quantification (I) illustrated. Values represent means ± SEM; *P < .05; **P < .01. Weights (J) and lengths (K) of thrombi that developed in the IVC 48 hours after ligation were measured in 4- and 16-month-old mice administered NAC for 2 months (n = 6). Values represent means ± SE; **P < .01.
H2 O2 contributes to mTORC1-dependent platelet activation during aging. FACS analysis of platelets (A) and MKs (B) from 4- or 16-month-old mice for intracellular H2O2 levels (n = 10). MFI values represent means ± SEM; **P < .01. (C) Western blot analysis of mouse platelets from 4- and 16-month-old WT or Raptor KO mice stimulated with 50 μM H2O2 for 30 minutes for mTORC1 activity. (D) Complete blood count analysis of 4- and 16-month-old mice administered NAC for 2 months (n = 10). MPV and platelets are shown. Values represent means ± SEM; *P < .05; **P < .01. (E) VWF IHC staining of femur bone sections from mice described in panel D. (F) Quantification of MK size is illustrated. Values represent means ± SEM; *P < .05. (G) PF4 ELISA analysis of plasma from mice described in panel D. Values represent means ± SEM; **P < .01. FACS analysis (H) of platelets incubated with 0.2 U/mL thrombin for 15 minutes at room temperature from mice described in panel D for integrin αIIbβ3 activation (n = 10) and quantification (I) illustrated. Values represent means ± SEM; *P < .05; **P < .01. Weights (J) and lengths (K) of thrombi that developed in the IVC 48 hours after ligation were measured in 4- and 16-month-old mice administered NAC for 2 months (n = 6). Values represent means ± SE; **P < .01.
Discussion
The results of the present study revealed an essential role of mTORC1 signaling in aging-related platelet formation and VTE. A number of findings highlight the utility of the mTORC1 pathway as a potential therapeutic target for VTE: (1) mTORC1 activities in MKs and platelets increased with aging; (2) chemical inhibition or genetic deletion of MK and platelet mTORC1 reduced the susceptibility of aged mice to VTE; (3) inhibition of mTORC1 suppressed age-dependent elevation of MK and platelet sizes and platelet activation in mice; and (4) increased ROS levels during aging contributed to mTORC1 activation in MKs and platelets.
Platelets secrete and express a number of substances that act as crucial mediators of coagulation and thrombosis.39 Platelet activation occurs in patients with DVT.6-8 Although conflicting results have been reported,40 the majority of studies to date have highlighted an increase in platelet size with aging,13 providing a potential explanation for higher thrombotic risk in aged individuals.3-5 As larger platelets contain more granules and express procoagulant surface proteins and adhesion molecules, they display greater prothrombotic potential.5,12 Established cardiovascular risk factors, such as smoking, diabetes mellitus, hypertension, hypercholesterolemia, and obesity, can also influence MPV,41 indicating an association of MPV with thrombosis. Data from the current study confirmed elevation of MPV with aging in mice. Although the platelet production rate and MPV are physiologically correlated, unlike MPV, we did not observe a positive correlation of platelets with mTORC1 activity and thrombosis in the experimental mice. An inverse relationship between platelets and MPV in physiological and specific pathological conditions has been frequently described, with lower platelet counts and higher MPV reported in elderly patients.42 However, we observed elevation of both MPV and platelets in aged mice, indicating species differences in platelets, but not MPV. The changes and significance of platelets in age-related thrombosis need to be further defined. Considering that increased MPV is a predictor of cardiovascular risk and unprovoked VTE,14,15 and MPV assessment is a simple method that yields results within a short time with low cost and easy detection in routine blood tests, it is highly likely that application of the universally acceptable standards of MPV measurement will allow the utilization of this tool in large prospective studies on conditions associated with thrombosis.
Platelets may play differential or even opposite roles in arterial and VTE.43,44 Previous reports on the role of mTORC1 in thrombosis have focused on the consequence of rapalog use in patients receiving organ transplants or implantation of rapamycin-eluting stents on artery thrombosis, with conflicting results. For instance, hepatic artery thrombosis was observed shortly after liver transplantation in patients treated with rapamycin, and subacute coronary artery thrombosis induced after the implantation of rapamycin-eluting stents.29,30 Rapamycin was shown to induce tumor-specific thrombosis but had no effect on normal tissues.45 In contrast, other studies demonstrated that rapalog use was safe and not associated with an increased risk of arterial or VTE.27,28,32 Mural thrombi are formed by circulating platelets interacting with activated endothelial cells or the subendothelial matrix. The specific roles of platelets or endothelial cell mTORC1 in thrombosis have not been elucidated. Our experiments using mice with platelet-specific deletion of mTORC1 clearly demonstrate that platelet mTORC1 promotes VTE. Rapamycin reduces thrombosis in old, but not young, mice. We speculate that the insensitivity of young mice to rapamycin may result from the following: (1) the low basic levels of mTORC1 activity and platelet activation, and small thrombus size formed in young mice, and rapamycin treatment might not further significantly reduce the thrombus size in these mice; (2) the effect of rapamycin treatment on platelets is not as efficient as platelet-specific Raptor deletion in which mTORC1 activity was completely wiped out; and (3) rapamycin might have adverse effects on other cells, such as monocytes, endothelial cells, and neutrophils, which are directly or indirectly involved in this process. Also, rapamycin did not have a significant effect on tBT in mice (supplemental Figure 14). It would be interesting to establish the role of endothelial mTORC1 in venous and arterial thrombosis using mice with endothelial cell–specific deletion of Raptor.
A major determinant of platelet production is MK size, which is regulated both by ploidization and increase in cytoplasmic volume at the end of maturation.42 Previous in vitro studies using rapamycin established an essential role of mTORC1 in platelet production and activation.23-26 Blockage of mTORC1 by rapamycin decreases MK cell size, suppresses proliferation, proplatelet formation, polyploidization, and maturation of MKs, indicating a crucial role of mTORC1 in megakaryocytopoiesis.26 Rapamycin also inhibits platelet spreading and Rac1-driven platelet activation and aggregation.25 The changes in mTORC1 activity and platelet production with aging are currently unknown. Here, we revealed for the first time that both MK size and MPV are elevated during aging, in correlation with increased mTORC1 activity in MKs and platelets. Inhibition of mTORC1 with either rapamycin or Raptor deletion reduced age-related elevation of MK size and MPV. Consistent with previous findings,22 rapamycin had no effect on elevated platelet numbers in aged mice. Although the precise molecular mechanisms remain to be identified, our results clearly suggest that increased mTORC1 activity contributes to age-dependent changes in platelet production, including elevation of MK size and MPV.
Aging is accompanied by a generalized increase in oxidative stress, and increased generation of ROS may contribute to cardiovascular events, including thrombosis. ROS are produced within the vascular system by monocytes, endothelial cells, neutrophils, and platelets,46 and ROS formation is functionally relevant for platelet activation.37 In addition, low doses of ROS may act as mitogenic factors to stimulate cell growth and proliferation. Data from our study indicate that the age-related increase in ROS promotes MK growth and platelet production via an mTORC1-dependent pathway, because ROS stimulated mTORC1 in MKs and inactivation of mTORC1 reduced MK size and MPV in aged mice. It is widely believed that aging is caused by accumulation of random molecular damage because of ROS. Interestingly, recent evidence supports an alternative explanation. Aging is driven by mTOR, as mTORC1 overactivation causes increased mitochondrial biogenesis and accumulation of higher levels of ROS.47 The age-related increase in ROS is reduced by inhibition of mTORC1, supporting a positive feedback mechanism regulating age-related changes in MKs and platelets. Specifically, mTORC1 hyperactivation leads to enhanced production of ROS, which, in turn, stimulate mTORC1 activity.
In summary, increased ROS production with aging activates mTORC1 in MKs and platelets, which, in turn, enhances MK and platelet sizes and platelet activation to promote aging-related VTE. Identification of the involvement of mTOR signaling in mediating VTE may have important pharmacotherapeutic implications for patients with VTE and other diseases characterized by age-related hyperactivation of platelets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the State Key Development Program for Basic Research of China (2013CB945203 and 2015CB55360) (X.B.) and the National Natural Sciences Foundation of China (grants 81571389 [L.W.], C110305 [M.X.], U1301222 [X.B.], and 81530070 [X.B.]).
Authorship
Contribution: J.Y., X.Z., X.F., and M.X. performed experiments and analyzed data; D.Y., B.L., M.D., L.S., J. Lu, Z.L., R.L., J. Liu., L.W., and M.Z. collected samples and performed experiments; J.Y., M.X., and X.B. wrote the manuscript; and Y.J., and X.B. supervised the project and designed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaochun Bai, Department of Cell Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China; e-mail: baixc15@smu.edu.cn.
References
Author notes
J.Y., X.Z., and X.F. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal