In this issue of Blood, Brauer et al model altered T-cell development of severe combined immunodeficiency (SCID) and Omenn syndrome (OS) patients with human induced pluripotent stem cells (iPSCs), and reveal important differences in T-cell receptor (TCR) repertoire diversity that help to understand the disparity of clinical and immunologic phenotypes that result from distinct RAG1 mutations.1
Modeling altered T-cell development of SCID and OS patients with dermal fibroblast-derived iPSCs.
Modeling altered T-cell development of SCID and OS patients with dermal fibroblast-derived iPSCs.
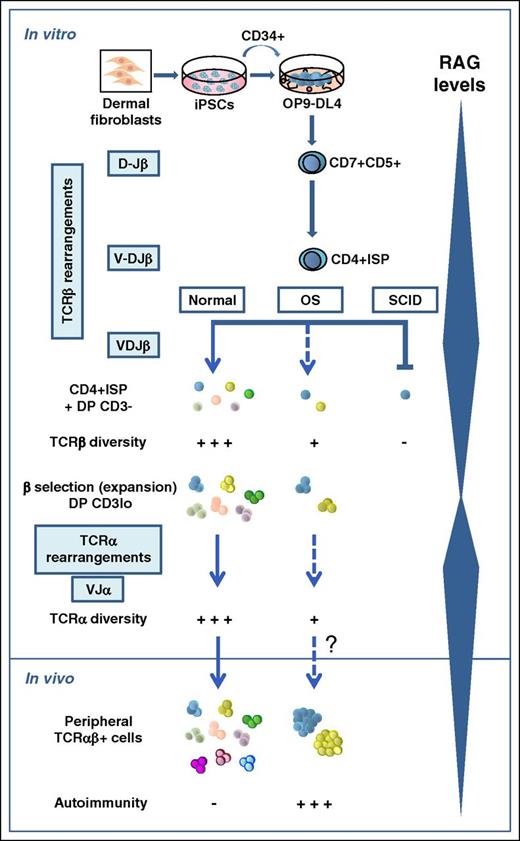
T and B lymphocytes are critical components of adaptive immunity, capable of recognizing millions of antigens with their surface antigen receptors (TCR and B-cell receptor, respectively). Their variable regions are encoded by exons assembled during lymphocyte development from the combinatorial joining of one of multiple germ line variable (V), diversity (D), and joining (J) gene segments.2 VDJ recombination is thus of fundamental importance for the generation of diverse antigen receptor repertoires and is absolutely dependent on the synergistic activity of the recombination-activating genes 1/2 (RAG1/2),3 which are regulated in a lymphoid and developmental stage-specific context. In humans, mutations of the RAG genes are associated with distinct clinical and immunologic phenotypes, which reflect the severity of the VDJ recombination defect and are characterized by variable association to infections and/or autoimmunity.4 Null RAG mutations totally abolish the VDJ recombination and result in a complete block of B- and T-cell development at the progenitor stage and thus in SCID, which represents approximately half of the human T−B− SCIDs. In contrast, hypomorphic RAG mutations support modest, but residual, recombination activity and cause OS, a rare autosomal recessive SCID associated with an oligoclonal expansion of autoreactive peripheral T cells that infiltrate and damage peripheral organs, whereas B cells are typically absent.4
Progress in our knowledge of the molecular mechanisms accounting for the phenotypic heterogeneity of human RAG deficiency derives from recent advances in structural modeling of the RAG complex and the development of novel cellular platforms to measure the expression and function of mutant RAG proteins in vitro.4 However, the functional impact of the diverse RAG mutations on T-cell development cannot be retrospectively studied in patients, and thus the developmental pathophysiology of the resulting disease remains ill-defined. To fill this gap, Brauer et al have now taken advantage of recent studies showing that T cells can be generated in vitro from iPSCs cocultured onto OP9 stromal cells expressing the delta-like 1 (DL-1) Notch ligand,5 and demonstrating that the model faithfully recapitulates the early T-cell developmental arrest that suffer X-linked SCID patients carrying an IL2RG mutation.6 Brauer et al extend this work to RAG1 mutant patients and validate the suitability of the model to elucidate the causality of SCID vs OS phenotypes. Their assay recapitulates T-cell developmental transitions in CD34+ hematopoietic progenitors obtained from SCID or OS dermal fibroblast-derived iPSCs harboring null or hypomorphic RAG1 mutations, which support undetectable or very modest (3.5%) recombination activity in vitro, respectively. The system also allows tracing TCR repertoire diversity generated at sequential stages of normal and defective T-cell development (see figure).
Development of αβ T cells is a complex multistep process that involves the sequential rearrangement of genes encoding the β and α chains of the TCR at 2 consecutive developmental checkpoints characterized by maximal expression levels of RAG genes.7 In humans, DJ rearrangements at the TCRB locus first occur in CD7+CD5+ pro-T cells at the CD4−CD8− double-negative thymocyte stage, whereas VDJ TCRB gene rearrangements and expression of full-length TCRB transcripts start in CD4+CD8− immature single-positive (ISP) (ie, CD4+ISP) thymocytes, and become common in a downstream CD4+CD8+ double-positive (DP) thymocyte subset that is highly enriched in large cycling cells expressing low CD3 levels (CD3lo), characteristic of pre-TCR+ cells. Therefore, the ISP to DP CD3lo transition represents the critical checkpoint at which developing thymocytes with a successful TCRB gene rearrangement downregulate RAG expression and undergo β-selection by signaling through the pre-TCR heterodimer (TCRβ/pTα), which promotes the clonal expansion of DP CD3lo pre-T cells.8 Thereafter, DP thymocytes downregulate the pre-TCR, stop cycling, and re-express RAG genes to induce TCRA gene rearrangement and finally TCR-αβ expression.9
Brauer et al have directly addressed when and how this developmental pathway is altered in OS and SCID patients by coculturing their iPSC-derived CD34+ progenitors on OP9 cells expressing the DL-4 Notch ligand (OP9-DL-4). The authors show that the critical transition from CD4+ISP to DP CD3lo pre-T cells observed in control cultures is severely impaired for both SCID and OS. Accordingly, no patient DP cells with a CD3lo phenotype characteristic of pre–TCR-expressing pre-T cells are expanded in vitro, confirming a specific blockade at the β-selection checkpoint, which concurs with the severe lymphoid depletion observed in the thymus of RAG-mutant patients. However, both OS and SCID CD7+CD5+ pro-T cells produce in vitro considerable, yet reduced, numbers of CD4ISP and DP CD3− pre-T cells with no detectable intracellular TCR-β expression, which show a drastically reduced proportion of TCRB (and TCRA) rearrangements and a severe restriction of TCRB (and TCRA) repertoire diversity, with preferential usage of few VDJ genes, compared with controls. In the case of SCID samples, RAG1 activity is severely affected in vitro, and extremely rare cells are thus expected to accomplish functional TCRB rearrangements and pre–TCR-mediated β-selection. OS pre-T cells, in contrast, contain residual RAG activity that supports some DNA recombination and a higher propensity for DNA breaks. Unexpectedly, they do not display an enhanced ability to progress beyond the β-selection checkpoint when compared with SCID cells in vitro, likely because, as the authors suggest, most of them accumulate single-strand DNA breaks that eventually may lead to cell death. In vivo, however, a few OS pre-T cells must succeed at some TCRB rearrangement, traverse the β-selection checkpoint to accomplish TCRA rearrangement, and migrate to the periphery as an oligoclonal population of T cells with a restricted TCR-αβ repertoire that may escape central tolerance, as such cells are readily detected and have been shown to contribute to autoimmunity in OS patients.4
Although the OP9-DL-4 system could not support the expansion and developmental progression of OS surviving thymocytes, it served to demonstrate their unique differences in VDJ diversity that may explain the phenotypic diversity of RAG1-associated immunodeficiency (ID). This highlights the applicability of iPSCs to model altered early T-cell development in human ID.
Conflict-of-interest disclosure: The author declares no competing financial interests.