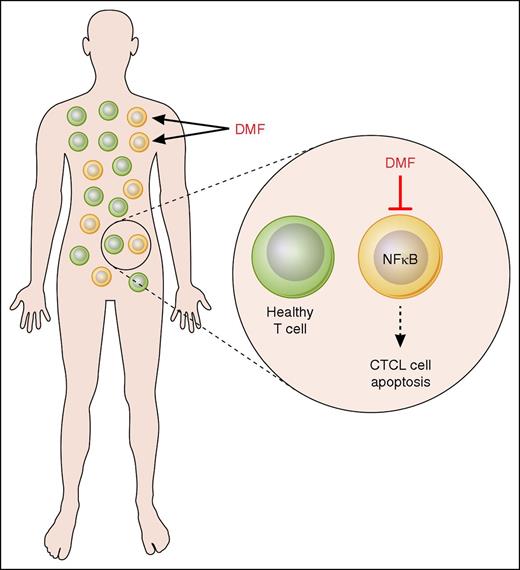
In this issue of Blood, Nicolay et al present their exciting findings that show that dimethyl fumarate (DMF) specifically inhibits NF-κB activity in cutaneous T-cell lymphoma (CTCL) cells, thus inducing their apoptosis, while having minimal effect on healthy T cells (see figure). Their study suggests that DMF may represent a new promising therapeutic option in CTCL.1
Mechanism of DMF action in CTCL. DMF specifically inhibits NF-κB activity in CTCL cells, thus inducing their apoptosis, while having minimal effect on healthy T cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mechanism of DMF action in CTCL. DMF specifically inhibits NF-κB activity in CTCL cells, thus inducing their apoptosis, while having minimal effect on healthy T cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
CTCL represents a heterogeneous group of rare lymphoproliferative disorders that are characterized by monoclonal proliferation of malignant T lymphocytes primarily homing to the skin.2 Several therapeutic options exist, but none of these represent a curative approach.3,4 Therefore, there is an urgent need for the development of novel therapeutic options with higher efficacy rates, curative potential, and milder toxicity profiles.
A characteristic feature of the malignant T cells in CTCL is their resistance toward cell death due to the constitutive activation of the transcription factor NF-κB.5-7 In addition to inducing expression of antiapoptotic genes, NF-κB induces expression of immunosuppressive genes in CTCL cells,8,9 thus inhibiting immune responses and contributing to the immunosuppressive nature of CTCL. Therefore, NF-κB represents an important therapeutic target in CTCL, as an NF-κB–directed therapy would leave bystander T cells largely unaffected.
DMF is a potent inhibitor of NF-κB signaling in activated cells, whereas it has a marginal effect on resting cells. It has been approved and clinically used for the treatment of psoriasis and multiple sclerosis.
DMF has relatively mild side effects, which makes it a fairly well-tolerated drug. This is especially attractive for the clinical prospects of DMF as a potential medication for CTCL because many other treatment options are limited by acute or cumulative toxicity or adverse effects. Against this background, Nicolay et al investigated the effects of DMF on CTCL cells. The rationale of this study was to use the NF-κB inhibitory properties of DMF to restore the apoptotic sensitivity in malignant T cells. Indeed, using a combination of elegant in vitro and in vivo experiments, the results of Nicolay et al suggest that DMF may successfully expand the spectrum of systemic treatment options for CTCL.
First, the authors showed that DMF induces cell death in CTCL patient-derived CD4 cells as well as in CTCL cell lines, whereas it has a minimal effect on control T cells. Their in vitro results indicate that the mechanism responsible for the DMF-induced apoptosis in CTCL cells consists of the inhibition of NF-κB activity and NF-κB–dependent transcription. Interestingly, 1 of the genes that was most inhibited by DMF was the NF-κB–regulated proto-oncogene Bcl3.1 Bcl3 is highly expressed in CTCL cells, where it has a prosurvival and immunosuppressive function.10 Therefore, the findings of Nicolay et al indicate that the DMF-mediated Bcl3 suppression potentiates the DMF-mediated NF-κB inhibition, thus enhancing the DMF-induced cell death in CTCL cells.
To study the impact of DMF in vivo, the authors then developed 2 CTCL xenograft mouse models with different cutaneous localizations of the T-cell infiltrate, and showed that both oral and parenteral DMF treatment reduces the tumor growth. The oral effectiveness of DMF underlines the clinical relevance of this study as DMF has been used only as an oral, but not as a parenteral, medication. In addition, DMF prevented the CTCL tumor spreading to distant organs, and enhanced cell death in primary tumors as well as in distant metastases, leaving the surrounding tissues widely unaffected. None of the mice experienced severe side effects by the DMF treatment; this underscores the excellent tolerability of the drug due to the high specificity of the DMF-induced cell death on malignant T cells.
Can DMF become the new therapeutic option in CTCL? DMF restores apoptosis in CTCL cells by inhibiting the constitutive activity of NF-κB, while having a minimum effect on healthy T cells. In the xenograft mouse models, the DMF-induced apoptosis of CTCL cells does not only inhibit tumor growth, but also prevents tumor spreading from skin to distant organs. This is highly important, as containment of CTCL cells in the skin is associated with a more favorable prognosis. The oral effectiveness of DMF may be quickly translated into oral CTCL treatment. These features of DMF render it a promising drug with few side effects; DMF may be better tolerated than most established CTCL treatment regimens. Altogether, the findings by Nicolay et al strongly suggest a prompt evaluation of DMF treatment in CTCL patients in a clinical trial.
Conflict-of-interest disclosure: The author declares no competing financial interests.