Key Points
DMF induces specific cell death in CTCL cells and inhibits CTCL tumor growth and metastasis in vivo via inhibition of NF-κB.
DMF therefore represents a promising, nontoxic novel therapeutic approach to treating CTCL.
Abstract
Despite intensive efforts in recent years, a curative therapy for cutaneous T-cell lymphoma (CTCL) has not yet been developed. Therefore, the establishment of new therapeutic approaches with higher efficacy rates and milder side effects is strongly desired. A characteristic feature of the malignant T-cell population in CTCL is resistance toward cell death resulting from constitutive NF-κB activation. Therefore, NF-κB–dependent cell death resistance represents an interesting therapeutic target in CTCL because an NF-κB–directed therapy would leave bystander T cells widely unaffected. We investigated the effects of dimethyl fumarate (DMF) on CTCL cells in vitro and in vivo. DMF induced cell death in primary patient-derived CD4+ cells and CTCL cell lines, but hardly in T cells from healthy donors. DMF-induced cell death was linked specifically to NF-κB inhibition. To study the impact of DMF in vivo, we developed 2 CTCL xenograft mouse models with different cutaneous localizations of the T-cell infiltrate. DMF treatment delayed the growth of CTCL tumors and prevented formation of distant metastases. In addition, DMF induced increased cell death in primary CTCL tumors and in liver metastases. In summary, DMF treatment represents a remarkable therapeutic option in CTCL because it restores CTCL apoptosis in vitro and in preclinical models in vivo and prevents spreading of the disease to distant sites. DMF treatment is of particular promise in CTCL because DMF is already in successful clinical use in the treatment of psoriasis and multiple sclerosis allowing fast translation into clinical studies in CTCL.
Introduction
Cutaneous T-cell lymphoma (CTCL) includes a heterogeneous group of rare lymphoproliferative disorders that are characterized by monoclonal proliferation of T lymphocytes primarily homing to the skin.1 Other organs can be affected secondarily. Several therapeutic options exist for treatment of CTCL, but none represent a curative approach.2-4 Furthermore, CTCL therapy is often complicated by high relapse rates, despite application of highly efficient cytoreductive or immunomodulatory treatment regimens and by severe side effects and toxicities. Therefore, there is an urgent need for the development of novel therapeutic options with higher efficacy rates, curative potential, and milder toxicity profiles.
Several alterations of cellular and molecular signals have been described that may add to transforming normal T cells into malignant CTCL cells, but many steps in this cascade remain elusive.5-7 It is, however, well-established that the malignant potential of CTCL depends on its distinct cell death resistance phenotype rather than on hyperproliferation. CTCL resistance toward cell death stimuli also complicates therapy because most cancer treatments aim at induction of apoptosis. Among several other factors that account for resistance toward apoptosis, CTCL cells show constitutive activation of the transcription factor NF-κB.8,9 NF-κB is also known to act as a pro-survival factor and to contribute to cell death resistance in various hematological malignancies.10-12 In CTCL cells, inhibition of NF-κB induced apoptosis in vitro.9 All NF-κB inhibitors used so far however have been found to be toxic and not applicable for therapeutic use.9 For these reasons, NF-κB remains an attractive therapeutic target in CTCL, whereas its pharmacological manipulation still poses major challenges to be overcome. Mechanistically, constitutive NF-κB activity in CTCL cells can be caused by different genetic alterations. Recently, a defect in the phosphatase PP4R1 was identified in CTCL cells.13 Lack of PP4R1 expression disrupts the assembly and inhibitory activity of a PP4c holoenzyme, impairing the deactivation of NF-κB signaling.13 In addition, amplifications and activating mutations in the CARD11 and the TNFRSF1B gene encoding the tumor necrosis factor receptor 2 (TNFR2) were identified in up to 30% of patients with high-stage CTCL.14-16 These mutations cause constitutive signaling through the noncanonical NF-κB pathway in CTCL cells, further enhancing their cell death resistance.
The small compound dimethyl fumarate (DMF) can unfold a wide variety of effects on cellular signaling, cell death, and proliferation.17-20 In particular, DMF is a potent inhibitor of NF-κB signaling in activated T cells21 and different malignant cells such as melanoma and glioblastoma cells.22-24 DMF has almost no apoptotic effect on resting T cells or other bystander cells, which correlates with the fact that they do not show elevated NF-κB activity.21 Consequently, marked clinically apparent immunosuppression usually does not result from DMF treatment, despite its pleiotropic cellular effects.25-27 For diseases such as psoriasis and multiple sclerosis, DMF is approved and clinically used.21-23 The drug has also shown favorable effects in off-label treatment of a wide variety of inflammatory and immunological diseases.25 In addition, DMF is characterized by a profile of rather mild side effects, which makes it a fairly well-tolerated drug. This is especially attractive for the clinical prospects of DMF as a potential medication for CTCL because many other oncological treatment options are limited by acute or cumulative toxicity or adverse effects.27
Against this background, we studied the effects of DMF on CTCL cells in vitro and in vivo. The rationale of this study is to make use of the NF-κB inhibitory properties of DMF on malignant T cells to restore apoptotic sensitivity. Indeed, our results suggest that DMF may successfully expand the spectrum of systemic treatment options for CTCL.
Materials and methods
Patients
Ten patients with Sézary syndrome (CTCL stage IV) diagnosed according to World Health Organization-European Organization of Research and Treatment of Cancer classification of CTCL and criteria of the international society of cutaneous lymphomas were included in the study.1 As controls, we investigated blood samples of healthy donors (n = 10) (see supplemental Table 1, available on the Blood Web site). Informed consent was obtained from all subjects before inclusion. The study was conducted according to ethical guidelines at our institution and the Helsinki Declaration and was approved by the ethics committee II of the University of Heidelberg.
Reagents and T cells
DMF, monomethyl fumarate (MMF), and propidium iodide were purchased from Sigma. Annexin V–fluorescein isothiocyanate antibody was obtained from Immunotools, growth factor reduced Matrigel from Becton Dickinson, fluorescent dyes dichlorodihydrofluorescein-diacetate, tetramethylrhodamine ethyl ester, and 5,5,6,6-tetrachloro-1,1,3,3 tetraethylbenzimidazolyl carbocyanine from Fisher Scientific.
Cell death assays, determination of the mitochondrial membrane potential, NFκB luciferase reporter assay, quantitative reverse transcription (qRT) polymerase chain reaction (PCR), western blot, and enzyme-linked immunosorbent assay (ELISA) were performed as described elsewhere.9,13,28-33 A brief description of these methods is provided in the supplemental data.
NF-κB subunit nuclear binding ELISA
For quantification of NF-κB activity, DMF-treated cells were harvested after 1 and 16 hours. Nuclear extracts were prepared with the Nuclear Extract Kit (Active Motive) according to manufacturer’s instructions with the exception of using lysis buffer from the TransAM Family Transcription Factor Assay Kit (Active Motive).
Protein concentration of nuclear extracts was measured with Pierce BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer’s recommendations; 2 µg of nuclear extracts were then analyzed for DNA-binding activity of p50, p65, and RelB using the TransAM NF-κB Family Transcription Factor Assay Kit (Active Motive).
In vivo xenograft CTCL mouse model and DMF treatment
All animal experiments were in accordance with the approved guidelines of the local Governmental Committee for Animal Experimentation (RP Karlsruhe, Germany, licenses G142/11 and G40/14). Mice were maintained at a 12-hour light-dark cycle with unrestricted diet and water. Under isoflurane inhalation anesthesia (1% to 1.5% in O2, 0.5 L/min), 1 × 106 HH cells suspended in 30 µL of phosphate-buffered saline (PBS)/Matrigel (1:1, vol/vol) were injected intradermally into the right flank of 9-week-old female nonobese diabetic severe combined immunodeficiency γ (NSG) mice recruited from the Center for Preclinical Research, DKFZ, Heidelberg, and kept on Kliba diet 3307 with n = 37 randomized mice/group. DMF was injected intraperitoneally (IP) (30 mg/kg bodyweight, dissolved at 3 mg/mL in 30°C prewarmed PBS, 10 µL/g mouse) once daily for 26 days starting at day 4 after heterotransplantation. The control group received PBS (10 µL/g mouse). Alternatively, the HH tumor xenograft was induced by subcutaneous transplantation of 3 × 106 cells per 200 µL PBS/Matrigel (1:1 vol/vol) and DMF was applied once daily in 0.8% methylcellulose and 10% ethanol (100 µL/10 g mouse) at a concentration of 20 mg/kg by oral gavage for 21 days starting 7 days after inoculation of cells (n = 20). Controls (n = 20) received vehicle alone. In a blinded manner, tumor volume (V) was measured with a caliper 3 times a week and calculated according to the formula: V = (length [mm] × width [mm]2)/2. Necropsies were taken when 1 tumor diameter reached 1.5 cm. No mice were lost from adverse DMF effects.
For tissue staining, liver and a quarter of tumor was fixed in 4% PBS-buffered formaldehyde and embedded into paraffin according to routine procedures.
Histologic, immunohistochemical, and immunofluorescent stainings
Hematoxylin and eosin staining as well as immunohistochemistry were performed on 5-µm paraffin sections as described previously.34 For immunofluorescence staining of frozen Matrigel plugs, tissue sections were fixed with 2% paraformaldehyde, permeabilized in methanol, and blocked in 1% bovine serum albumin in PBS++ (1X PBS, 1 mM MgCl2, 0.1 mM CaCl2). Sections were incubated with primary antibodies (cleaved caspase 3 [Cell Signaling Technology], cleaved caspase 8 [Santa Cruz Biotechnology], CD3 [Abcam], caspase-8 [Santa Cruz], phospho-p65 [Ser536, Cell Signaling], fluorophore-conjugated secondary antibodies, and Hoechst 33342 [Life Technologies]) diluted 1:50 to 1:100 in 0.2% bovine serum albumin in PBS. Immunofluorescent stainings were analyzed by confocal microscopy (LSM700 or LSM710, Zeiss). To acquire mean fluorescence intensities, single fluorescence intensities of fluorophore-conjugated secondary antibodies in regions of interest were recorded by total fluorescence per field of view in a 12-bit mode and relative units were quantified and averaged.
Microscopy
For microscopy, the following Nikon objective lenses were used (Nikon, Tokyo, Japan): Nikon Plan Apoλ 2×/0.1 and Nikon Plan Apoλ 20×/0.75. Microscopy was performed at 22°C throughout with cryopreserved or paraffin sections. As fluorochromes phycoerythrin, fluorescein isothiocyanate, and 4′,6 -diamidino-2-phenylindole were used for red, green, and blue stainings, respectively. The micrographs were taken with a Nikon DS-Fi2 camera using the Nikon DS-L3 software.
Statistical analyses
Data are presented as the mean ± standard error of the mean. Two-sided tests were used throughout, and the differences were considered statistically significant at P < .05. Pairwise (univariate) comparisons were performed using Student t test or the Mann-Whitney U test as appropriate. For multivariate analysis, analysis of variance was used. Normalizations were performed as described in the figure legends and supplemental data.
Results
DMF induces cell death in patient-derived CTCL and CTCL cell lines
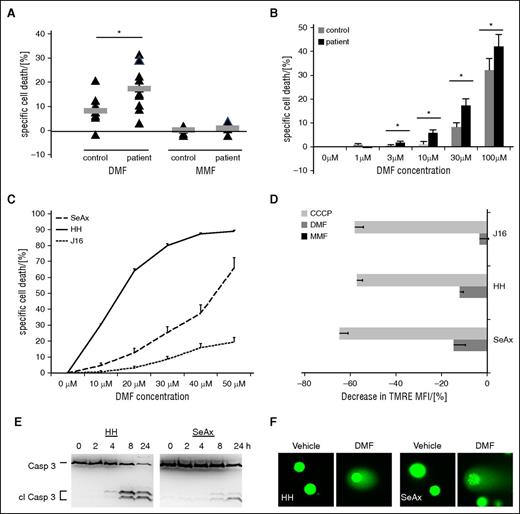
Primary CD4+ T cells were isolated from the peripheral blood of 10 patients with Sézary syndrome and of 10 age-matched healthy volunteers as controls. Upon DMF treatment of 48 hours, the patient-derived T-cell samples showed significantly higher rates of cell death compared with control T cells (Figure 1A). This effect was independent of the actual clinical staging of the patient as reflected by malignant cell count in the peripheral blood or by skin tumor burden as well as of prior therapies (Figure 1A; supplemental Table 1). To mimic physiologic conditions, DMF was solubilized at pH 7.4 in RPMI 1640 medium before treatment to allow partial hydrolysis, as it occurs in the organism.35 As expected, MMF, the partly hydrolyzed metabolite of DMF,36 failed to induce cell death (Figure 1A; supplemental Figure 1A). After 48 hours of DMF treatment, we found significantly increased cell death in patient-derived CD4+ T cells upon treatment with 3 µM of DMF, whereas 30 µM of DMF was needed to induce comparable cell death in CD4+ T cells from healthy donors indicating a broad therapeutic window for the use of DMF in CTCL (Figure 1B). Again, treatment of CTCL cells with different doses of MMF for 48 hours did not result in cell death in any group underlining the specificity of this effect of DMF (supplemental Figure 1B). As opposed to resting T cells (D0), activated T cells (D6) depend on NF-kB.37 Upon DMF treatment, activated, but not resting, healthy T cells were shown to undergo cell death, reflecting selective cell death induction by DMF in cells with high NF-κB activity18,21,37 (supplemental Figure 1C).
DMF causes cell death in primary CTCL cells and CTCL cell lines. (A) Specific cell death in primary CD4+ cells isolated from 10 healthy volunteers (control) and 10 patients with Sézary syndrome (patient) upon treatment with 30 µM DMF (left) or 30 µM MMF (right) for 48 hours. Triangles, single samples; gray bars, median. (B) Specific cell death rates in primary CD4+ cells isolated from 10 healthy volunteers (control) and 10 patients with Sézary syndrome (patient) upon treatment with different concentrations of DMF for 48 hours. (C) Specific cell death rates in J16, HH, and SeAx cells upon treatment with different concentrations of DMF solubilized in dimethyl sulfoxide for 24 hours (n = 4, each). (D) Decrease in tetramethylrhodamine ethyl ester (TMRE) mean fluorescence intensity in J16, HH, and SeAx cells upon treatment with either 30 µM DMF or MMF or 10 µM CCCP for 24 hours (n = 4, each). *P < .05. (E) Western blot analysis of caspase 3 cleavage in HH (left) and SeAx (right) cells after treatment with 50 µM DMF for the indicated time points. (F) Single cell gel electrophoresis of HH (left panels) and SeAx (right panels) cell upon treatment with either vehicle or 50 µM DMF for 8 hours.
DMF causes cell death in primary CTCL cells and CTCL cell lines. (A) Specific cell death in primary CD4+ cells isolated from 10 healthy volunteers (control) and 10 patients with Sézary syndrome (patient) upon treatment with 30 µM DMF (left) or 30 µM MMF (right) for 48 hours. Triangles, single samples; gray bars, median. (B) Specific cell death rates in primary CD4+ cells isolated from 10 healthy volunteers (control) and 10 patients with Sézary syndrome (patient) upon treatment with different concentrations of DMF for 48 hours. (C) Specific cell death rates in J16, HH, and SeAx cells upon treatment with different concentrations of DMF solubilized in dimethyl sulfoxide for 24 hours (n = 4, each). (D) Decrease in tetramethylrhodamine ethyl ester (TMRE) mean fluorescence intensity in J16, HH, and SeAx cells upon treatment with either 30 µM DMF or MMF or 10 µM CCCP for 24 hours (n = 4, each). *P < .05. (E) Western blot analysis of caspase 3 cleavage in HH (left) and SeAx (right) cells after treatment with 50 µM DMF for the indicated time points. (F) Single cell gel electrophoresis of HH (left panels) and SeAx (right panels) cell upon treatment with either vehicle or 50 µM DMF for 8 hours.
Experiments with CTCL cell lines corroborated these results. The CTCL cell lines SeAx and HH have been demonstrated by us to exhibit moderate and high constitutive NF-κB activation, respectively,13 whereas the non-CTCL, T-cell leukemia cell line Jurkat J16 does not exhibit constitutive NF-κB activity and served as a negative control. We exploited these different levels of NF-κB activation to illustrate the correlation between DMF effectiveness with regard to cell death induction and NF-κB activity. DMF caused a strong increase in cell death in HH cells (Figure 1C; supplemental Figure 1D). The effect of DMF in SeAx cells was substantially weaker, but was still significant compared with the minor effect on J16 cells when we used medium-solubilized DMF (supplemental Figure 1D). To rule out partial inactivation of the compound by hydrolysis before treatment, we also treated the cell lines with DMF solubilized in dimethyl sulfoxide (Figure 1C) and quantified the inhibitory concentrations of DMF needed for cell death induction. We observed stronger effects in cell death induction in the CTCL cell lines as compared with medium-solubilized DMF, whereas the effect on J16 cells was almost unchanged. The concentrations needed to kill 50% of the cells were 15.1 µM in HH and 44.3 µM in SeAx cells, which parallels their constitutive NF-κB activity (Figure 1C). In line with the data obtained in primary T cells, none of the cell lines used showed any cell death induction upon treatment with MMF (supplemental Figure 1E). The effect of DMF on mitochondrial membrane potential corroborated our findings because it represents an additional readout for cell death induction. In particular, we observed a marked breakdown of mitochondrial membrane potential in SeAx and HH cells (Figure 1D; supplemental Figure 1F), suggesting an apoptotic nature of the observed cell death. In J16 cells, only a minor effect on mitochondrial membrane potential was detected. In line with the results described previously, MMF treatment did not lead to depolarization of the inner mitochondrial membrane (Figure 1D; supplemental Figure 1F). According to the guidelines of the Nomenclature Committee on Cell Death, induction of apoptosis was confirmed by 2 other assays.38 Caspase 3 cleavage (Figure 1E) and DNA fragmentation (Figure 1F) measured by single cell gel electrophoresis confirmed apoptotic cell death induction by DMF in HH and SeAx cells.
DMF inhibits NF-κB activity and signaling in CTCL cells
DMF has been described to be an inhibitor of NF-κB in activated and malignant cells.21-24,36 The constitutive NF-κB activation of CTCL cells is crucial for survival and maintenance of cell growth.8,9 To provide insight into the mechanistic background of cell death induction by DMF, we analyzed its effect on NF-κB activity in CTCL cells. J16 and HH cells were transfected with a luciferase reporter construct. The reporter gene assay displayed in Figure 2A revealed a 50× higher NF-κB activity in unstimulated HH cells compared with J16 cells verifying constitutive NF-κB activation in CTCL cells. Upon treatment with 50 µM DMF for 24 hours, NF-κB activity in HH cells decreased drastically, but remained unchanged in J16 cells (Figure 2B). This effect is reflected on messenger RNA level by a distinct time-dependent decrease of IκBα messenger RNA, an NF-κB target gene, upon DMF treatment (Figure 2C). On protein level, the DMF-induced NF-κB inhibition is reflected by a decrease in NF-κB-dependent TNF-α secretion (Figure 2D) and reduction of IκBα protein level in HH cells (supplemental Figure 2A).
DMF inhibits NF-κB activity in CTCL cells. (A) Specific luminescence of the NF-κB luciferase assay in J16 and HH cells 24 hours after transfection without treatment (n = 3, each). (B) Specific luminescence of the NF-κB luciferase assay in J16 and HH cells 24 hours after transfection with and without treatment with 50 µM DMF for 24 hours. The specific NF-κB activity of untreated J16 cells was normalized to 1 and all other activities were normalized to that value (n = 3 each). (C) Relative IκBα expression measured by qRT-PCR in SeAx (upper) and HH (lower) cells 1 to 3 hours after treatment with 50 µM of either DMF or MMF, normalized to GAPDH (n = 3 each). (D) TNF-α concentration in supernatants of HH cells either untreated or treated with 50 µM DMF for 12 hours (n = 3 each). (E) Normalized nuclear p65 activity measured by binding ELISA in HH and SeAx cells upon treatment with different concentrations of DMF and MMF for 1 hour (upper) and 16 hours (lower), (n = 3 each). DMSO, dimethyl sulfoxide. (F) Relative changes in expression levels of NF-κB target genes measured by qRT-PCR array after 3 hours of treatment with DMF and MMF (30 µM each). The quantitative values are (expression [DMF treatment])/(expression(MMF treatment)). (n = 3, each) *P < .05.
DMF inhibits NF-κB activity in CTCL cells. (A) Specific luminescence of the NF-κB luciferase assay in J16 and HH cells 24 hours after transfection without treatment (n = 3, each). (B) Specific luminescence of the NF-κB luciferase assay in J16 and HH cells 24 hours after transfection with and without treatment with 50 µM DMF for 24 hours. The specific NF-κB activity of untreated J16 cells was normalized to 1 and all other activities were normalized to that value (n = 3 each). (C) Relative IκBα expression measured by qRT-PCR in SeAx (upper) and HH (lower) cells 1 to 3 hours after treatment with 50 µM of either DMF or MMF, normalized to GAPDH (n = 3 each). (D) TNF-α concentration in supernatants of HH cells either untreated or treated with 50 µM DMF for 12 hours (n = 3 each). (E) Normalized nuclear p65 activity measured by binding ELISA in HH and SeAx cells upon treatment with different concentrations of DMF and MMF for 1 hour (upper) and 16 hours (lower), (n = 3 each). DMSO, dimethyl sulfoxide. (F) Relative changes in expression levels of NF-κB target genes measured by qRT-PCR array after 3 hours of treatment with DMF and MMF (30 µM each). The quantitative values are (expression [DMF treatment])/(expression(MMF treatment)). (n = 3, each) *P < .05.
The NF-κB transcription factor family consists of 5 proteins in mammals—p65 (RelA), RelB, c-Rel, p105/p50, and p100/52—that form distinct transcriptionally active homo- and heterodimeric complexes. The transcriptionally active complex in the canonical NF-κB pathway is a p65/p50 heterodimer.39 Both in HH and SeAx cells, DNA binding of p50, p65, and RelB proteins was significantly inhibited after DMF treatment (Figure 2E; supplemental Figure 3). Cellular phospho-p65 (pp65) levels were mildly decreased in SeAx, but not in HH cells (supplemental Figure 2B), indicating a more complex mechanism besides inhibition of p65 phosphorylation to cause DMF-induced NF-κB blockage. Inhibition of NF-κB activity alters expression of NF-κB target genes. In a qRT-PCR array, we detected several NF-κB target genes that were downregulated already after 3 hours of DMF treatment. Downregulation was most pronounced in HH cells. Among the downregulated genes, the bcl-3 gene was most strongly downregulated both in SeAx and HH cells (Figure 2F). Bcl-3 exerts its influence on transcription via interaction with p5040,41 and is needed for CTCL survival.42 In other T-cell lymphoma types, Bcl-3 activity is increased by translocations and chromosomal gains, resulting in increased NF-κB activity.43,44 Therefore, Bcl-3 downregulation further contributes to NF-κB inhibition and DMF-induced cell death in CTCL cells. Recently, transforming growth factor-β (TGF-β) levels were found to be regulated by NF-κB in CTCL.33,45 Accordingly, we found TGF-β downregulation in the HH and SeAx cell lines upon treatment with DMF, further supporting DMF-induced NF-κB inhibition (supplemental Figure 4).45
DMF treatment inhibits CTCL tumor growth in subcutaneous and orthotopic xenograft mouse models
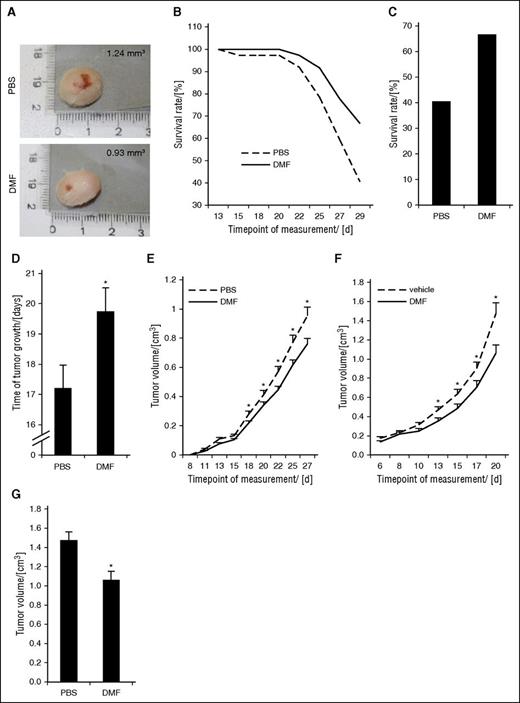
To confirm our findings in vivo, we established a subcutaneous and an intradermal orthotopic CTCL xenograft mouse model by injecting HH cells into NSG mice. The latter model closely represents CTCL cell localization in humans. At day 14 after tumor induction, the first animal in the PBS group of the intradermal model had to be euthanized. Figure 3A shows representative tumors of both groups after euthanizing of the animals. Figure 3B illustrates the survival rate of the mice in the DMF and control groups. Over time, the difference in survival between both groups increased, representing the antitumoral effect of DMF. At day 29 (ie, at the end of the treatment period), only 40% of the animals were alive in the control group, whereas almost 70% were still alive in the DMF group (Figure 3C). Moreover, none of the animals experienced weight loss or other severe side effects or died from the medication (supplemental Figure 5). On average, the time of tumor growth before reaching the limiting size of 1.5 cm in 1 dimension was significantly longer in the DMF group (19.75 ± 0.76 days) compared with the PBS group (17.22 ± 0.75 days) (Figure 3D). Figure 3E depicts the course of tumor growth in the 2 groups, showing significantly slower growth and smaller tumor volumes already after 18 days with a continuous increase of the difference between the 2 groups over time.
DMF treatment inhibits CTCL cell and tumor growth in subcutaneous and orthotopic xenograft mouse models. NSG mice were xenografted with HH cells intradermally and treated once daily with either 30 mg/kg bodyweight of DMF or PBS by IP injection. (n = 37 each). In the subcutaneous model, the NSG mice were xenografted with HH cells subcutaneously and treated once daily with either 20 mg/kg bodyweight of DMF or PBS orally by gavage (n = 20 each). (A) Macroscopic pictures of a representative primary HH tumor of the respective mouse from either a PBS-treated (upper) or a DMF-treated (lower) animal in the intradermal model. (B) Survival curves of NSG mice. Decrease in survival is either caused by spontaneous death or by reaching critical tumor size of 1.5 cm at the largest diameter. (C) Percent survival rate of orthotopically xenografted mice treated with PBS or DMF at day 29, the end of the treatment phase. (D) Median time of tumor growth in PBS- and DMF-treated intradermal CTCL xenograft mice from first detection of a tumor to either death or the end of the experiment. (E) Median tumor volume of PBS- and DMF-treated intradermal CTCL xenograft mice over time. (F) Median tumor volume of subcutaneous HH xenografts of mice treated with either 20 mg/kg bodyweight DMF or PBS by oral gavage (n = 20, each) over time. (G) Median tumor volume in PBS- and DMF-treated subcutaneous CTCL xenografts at day 20. *P < .05.
DMF treatment inhibits CTCL cell and tumor growth in subcutaneous and orthotopic xenograft mouse models. NSG mice were xenografted with HH cells intradermally and treated once daily with either 30 mg/kg bodyweight of DMF or PBS by IP injection. (n = 37 each). In the subcutaneous model, the NSG mice were xenografted with HH cells subcutaneously and treated once daily with either 20 mg/kg bodyweight of DMF or PBS orally by gavage (n = 20 each). (A) Macroscopic pictures of a representative primary HH tumor of the respective mouse from either a PBS-treated (upper) or a DMF-treated (lower) animal in the intradermal model. (B) Survival curves of NSG mice. Decrease in survival is either caused by spontaneous death or by reaching critical tumor size of 1.5 cm at the largest diameter. (C) Percent survival rate of orthotopically xenografted mice treated with PBS or DMF at day 29, the end of the treatment phase. (D) Median time of tumor growth in PBS- and DMF-treated intradermal CTCL xenograft mice from first detection of a tumor to either death or the end of the experiment. (E) Median tumor volume of PBS- and DMF-treated intradermal CTCL xenograft mice over time. (F) Median tumor volume of subcutaneous HH xenografts of mice treated with either 20 mg/kg bodyweight DMF or PBS by oral gavage (n = 20, each) over time. (G) Median tumor volume in PBS- and DMF-treated subcutaneous CTCL xenografts at day 20. *P < .05.
Independently, we generated a second xenograft model with subcutaneous HH cell injection. After 4 to 6 days, subcutaneous tumors were palpable. Because DMF is characterized by low bioavailability resulting from fast partial hydrolysis to MMF and binding to glutathione with inactivating effect,35,46 we applied DMF or vehicle as a control by oral gavage starting 7 days after tumor cell inoculation. Figure 3F shows the tumor growth curves of DMF and control groups. After 7 days of treatment the tumors in the DMF group were significantly smaller than in the PBS-treated group, an effect that increased over time. After 14 days of treatment, the tumors in the DMF-treated group were about 30% smaller than in the vehicle-treated group (Figure 3G).
DMF treatment induces cell death specifically within CTCL tumors in vivo
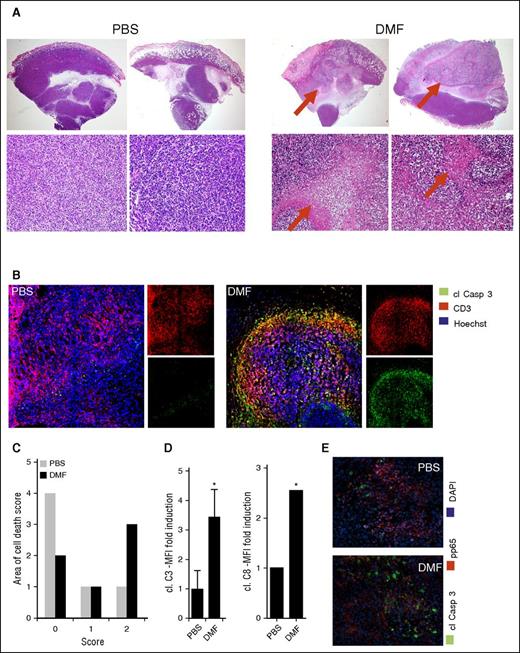
After the treatment phase in the intradermal xenograft model, the tumors and organs of the mice were collected, and hematoxylin and eosin-stained specimens were evaluated histologically (Figure 4A). Large and dense xenograft T-cell tumors were observed in the PBS-treated mice. The primary cutaneous tumors were characterized by a multinodular structure and consisted of very pleomorphic T cells with many mitoses. In contrast, many of the intradermal tumors of the DMF-treated mice showed large zones filled with necrotic T cells and cell debris. Some tumors were completely necrotic (Figure 4A, right). Furthermore, it was striking that the necrotic zones were observed exclusively in T-cell tumor areas, whereas the surrounding mouse tissue was not affected by necrotic cell death. Semiquantitative evaluation by using a necrosis score performed on 6 representative tumors of both mouse groups confirmed that cell death and necrosis were by far more pronounced in the CTCL tumors of DMF-treated animals compared with the PBS-treated control animals (Figure 4C).
DMF treatment induces massive cell death specifically within CTCL tumors in vivo. HH xenografted tumors grown intradermally in NSG mice that were treated once daily IP with either 30 mg/kg bodyweight DMF or PBS. (A) Representative hematoxylin and eosin–stained specimens of primary HH tumors (upper panels, 20×; lower panels, 200×). (B) Representative pictures of immunofluorescent stainings of primary HH tumors stained for cleaved caspase 3, CD3, and with Hoechst dye, the latter to counterstain nuclei. (C) Semiquantitative score of necrosis areas in the primary xenograft tumors (0% to 25% tumor area covered by necrosis = 0; 25% to 50% tumor area covered by necrosis = 1; 50% to 75% tumor area covered by necrosis = 2; 75% to 100% tumor area covered by necrosis = 3). (D) Quantification of cleaved caspase 3 (left) and cleaved caspase 8 (right) mean fluorescence intensity in HH tumors of either the PBS or DMF treatment group (n = 3 each). (E) Representative immunofluorescent pictures of primary HH xenograft tumors of a PBS- and a DMF-treated mouse stained for cl Casp3, p65, and with Hoechst dye. *P < .05.
DMF treatment induces massive cell death specifically within CTCL tumors in vivo. HH xenografted tumors grown intradermally in NSG mice that were treated once daily IP with either 30 mg/kg bodyweight DMF or PBS. (A) Representative hematoxylin and eosin–stained specimens of primary HH tumors (upper panels, 20×; lower panels, 200×). (B) Representative pictures of immunofluorescent stainings of primary HH tumors stained for cleaved caspase 3, CD3, and with Hoechst dye, the latter to counterstain nuclei. (C) Semiquantitative score of necrosis areas in the primary xenograft tumors (0% to 25% tumor area covered by necrosis = 0; 25% to 50% tumor area covered by necrosis = 1; 50% to 75% tumor area covered by necrosis = 2; 75% to 100% tumor area covered by necrosis = 3). (D) Quantification of cleaved caspase 3 (left) and cleaved caspase 8 (right) mean fluorescence intensity in HH tumors of either the PBS or DMF treatment group (n = 3 each). (E) Representative immunofluorescent pictures of primary HH xenograft tumors of a PBS- and a DMF-treated mouse stained for cl Casp3, p65, and with Hoechst dye. *P < .05.
This finding is corroborated by the measurement of caspase activity in the xenograft tumors of both groups. We stained tumors of 3 DMF- and 3 PBS-treated mice for CD3 as well as for cleaved caspases (clCasp) 3 and 8 of human and mouse origin to visualize apoptotic cell death in xenografted tumors as well as mouse host tissue (Figure 4B). Within the CD3+ T-cell tumors, clCasp3 was found in the DMF-treated animals, but was almost completely absent in the tumors of PBS-treated mice. A quantification of the green fluorescence intensity of the caspase staining revealed a 3.5-fold increase of clCasp3 and a 2.5-fold increase in cleaved caspase 8 in the xenograft tumors after DMF treatment (Figure 4D). These data not only show that the growth of CTCL tumors was inhibited in vivo in our mouse model, but also they confirm the in vitro data regarding DMF-induced CTCL cell death. We further performed double staining of clCasp3 and pp65 of the xenograft tumors. We found that CTCL cell nuclei were frequently positive for pp65 in the tumors of PBS-treated mice. clCasp3+ cells were very sparse, reflecting the low level of apoptotic cell death in the PBS-treated tumors (Figure 4E). In contrast, we found numerous clCasp3+ CTCL cells and hardly any pp65+ nuclei in the CTCL tumors of DMF-treated mice. Actually, we did not detect any double-positive cells in either group (Figure 4E). These results support the view that DMF induces cell death by inhibition of NF-κB in vivo.
DMF treatment inhibits metastasis of CTCL tumors in vivo
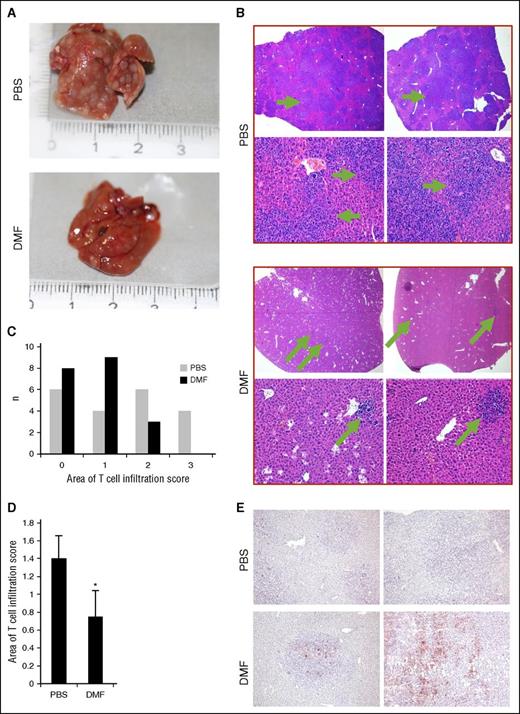
We examined livers of the intradermal xenografted mice to evaluate metastases of the T-cell tumors. Representative hematoxylin and eosin-stained livers of PBS- and DMF-treated mice of the xenografted tumor bearers are shown in Figure 5A. Multiple nodular areas of massive T-cell infiltrates were found in the PBS-treated animals that were positive for human CD45 (supplemental Figure 6), representing CTCL organ metastases. In the DMF-treated mice, however, macroscopic and microscopic metastasis occurred less frequently (Figures 5A-B). In addition, the degree of hepatic T-cell infiltration was reduced in DMF-treated mice because both incidence and size of the infiltrated liver areas were smaller than in the PBS-treated group (Figure 5C-D). To quantify the size of the T-cell areas in the livers, we used a semiquantitative score determining the percentage of area covered by T cells. We found significantly more livers with less than 50% of hepatic tissue infiltrated by T cells in the DMF-treated group as compared with the PBS-treated controls (Figure 5C). In contrast to PBS-treated animals, no livers of DMF-treated animals had more than 75% of hepatic tissue infiltrated with T cells. This translated into a significantly smaller median hepatic T-cell infiltration score in 20 DMF-treated animals compared with 20 PBS-treated animals (Figure 5D). A similar effect was observed for splenic metastases. We found xenograft T-cell infiltrates in all of the examined spleens of PBS-treated mice (n = 5), but only in 1 of 5 DMF-treated mice (supplemental Figure 7).
DMF treatment inhibits metastasis of CTCL tumors in vivo. Livers were collected from orthotopically HH xenografted tumor bearers that were treated once daily IP with either 30 mg/kg bodyweight DMF or PBS as control (n = 37, each). (A) Representative macroscopic pictures of an explanted liver of the respective mouse from either a PBS-treated (upper) or a DMF-treated (lower) animal in the intradermal model. (B) Representative pictures of hematoxylin and eosin–stained liver specimens (upper panels, 20×; lower panels, 200×). (C) Semiquantitative score of HH T-cell infiltrate areas within the mouse livers (0% to 25% liver area covered by T cells = 0; 25% to 50% liver area covered by T cells = 1; 50% to 75% liver area covered by T cells = 2; 75% to 100% liver area covered by T cells = 3). (D) Median of the semiquantitative score of HH-T cell infiltrates in liver of DMF- and PBS-treated HH xenograft tumor bearers. (E) Representative picture of liver specimens derived from DMF- and PBS-treated HH xenograft tumor bearers immunohistochemically stained for cleaved caspase 3. Note the positive staining in T-cell foci of DMF-treated tumor bearers, but not in surrounding host tissue. *P < .05.
DMF treatment inhibits metastasis of CTCL tumors in vivo. Livers were collected from orthotopically HH xenografted tumor bearers that were treated once daily IP with either 30 mg/kg bodyweight DMF or PBS as control (n = 37, each). (A) Representative macroscopic pictures of an explanted liver of the respective mouse from either a PBS-treated (upper) or a DMF-treated (lower) animal in the intradermal model. (B) Representative pictures of hematoxylin and eosin–stained liver specimens (upper panels, 20×; lower panels, 200×). (C) Semiquantitative score of HH T-cell infiltrate areas within the mouse livers (0% to 25% liver area covered by T cells = 0; 25% to 50% liver area covered by T cells = 1; 50% to 75% liver area covered by T cells = 2; 75% to 100% liver area covered by T cells = 3). (D) Median of the semiquantitative score of HH-T cell infiltrates in liver of DMF- and PBS-treated HH xenograft tumor bearers. (E) Representative picture of liver specimens derived from DMF- and PBS-treated HH xenograft tumor bearers immunohistochemically stained for cleaved caspase 3. Note the positive staining in T-cell foci of DMF-treated tumor bearers, but not in surrounding host tissue. *P < .05.
In addition, we immunostained livers for clCasp3 to check for an influence of DMF not only on the development of liver metastasis, but also on cell death within metastases. Multiple clCasp3+ cells were localized within the foci of T-cell infiltrates in DMF-treated mice representing apoptotic cells, whereas almost no positive cells were present in PBS-treated animals (Figure 5E). These results confirm that cell death is enhanced by DMF in vivo both in primary CTCL tumors and in distant metastases. The lack of caspase 3 cleavage in the surrounding mouse hepatocytes underlines the specificity of DMF-induced cell death in malignant T cells, leaving nonmalignant tissues and cells widely unaffected.
Discussion
Prior studies have tried to target the NF-κB pathway in CTCL, introducing a wide variety of drugs that influence NF-κB activity such as curcumin, bortezomib, and nonsteroidal anti-inflammatory drugs.47-49 Yet, these drugs exert their effects on NF-κB indirectly by targeting other signaling pathways primarily. So far, none of these drugs has shown convincing therapeutic responses in the treatment of CTCL.
Drugs that directly target NF-κB have already shown highly effective cell death induction in CTCL in vitro, but their toxicity was too high to be used in humans.8,9 Therefore, our study expands the spectrum of promising candidate therapeutic agents in CTCL. It adds DMF as a potential first treatment option that, besides indirect effects on NF-κB activation (eg, depletion of reduced Glutathione, blockage of Nrf2),50 also directly targets NF-κB signaling via inhibition of nuclear translocation of the subunits p50 and p65, which was already described in different cell types.36,51-53 Nevertheless, the exact mechanism is still not known. Furthermore, DMF may be safely used in a clinical setting because it is already used in psoriasis and multiple sclerosis treatment.25-27 Given its favorable profile regarding adverse events and the low risk of severe complications under proper therapy surveillance, DMF may even be superior to most established CTCL therapies with respect to severity of side effects. The only severe and irreversible side effect described for DMF is progressive multifocal leukoencephalopathy that can be almost completely ruled out by continuous blood count controls.54
The postulated mechanisms of action by which DMF may influence cell death include a wide variety of signaling pathways. DMF interferes with the glutathione system, heme oxygenase 1, and the Nrf2 pathway.46,55-58 Yet, the most prominent interaction in the induction of cell death by DMF is inhibition of NF-κB signaling18,21,51 ; however, the exact mechanism of DMF-induced NF-κB inhibition has not yet been completely elucidated. We showed that DMF treatment decreases DNA binding of the NF-κB subunit p65 and to a smaller extent p50 and RelB and thus impairs transcriptional activity of NF-κB. This is reflected by decreased expression of typical NF-κB target genes such as Iκbα or bcl-3. The massive decrease in bcl-3 gene expression upon DMF treatment is especially interesting for CTCL as Bcl-3 has been shown to be overexpressed in CTCL as a pro-survival factor. Suppression of Bcl-3 expression leads to cell death by an NF-κB–dependent pathway,42 indicating that decreased Bcl-3 expression potentiates the inhibition of the indispensable pro-survival factor NF-κB in a feed-forward cycle and thus enhances DMF-induced cell death in CTCL cells.
The evidence for effects of DMF on malignant cells is scarce. The drug gained broader clinical attention, however, when it was approved for MS therapy recently. In oncological therapy, evidence is limited to preclinical studies showing that DMF effectively inhibits melanoma growth and metastasis.22 To the best of our knowledge, our study is the first to examine the effect of DMF on a hematopoietic malignancy in vitro and in vivo. In particular, we provide evidence that the effects and mechanisms of DMF activity described for activated T cells are also valid for malignant T cells. This is plausible because they share many common signaling pathways. We conclude that the widely acknowledged anti-inflammatory effects of DMF may be exploited for antitumor effects in CTCL.
Our in vivo data corroborate the promising therapeutic effects of DMF in CTCL treatment. We showed that both oral and parenteral DMF treatment lead to reduced tumor growth and metastases in the CTCL xenograft mouse models. This underlines the clinical relevance of our findings because DMF is only used as an oral, but not as a parenteral, medication so far and may thus be quickly translated into an oral CTCL treatment. Furthermore, DMF cooperatively inhibits NF-κB and induces cell death specifically in CTCL cells, leaving the surrounding mouse tissues widely unaffected. None of the mice experienced severe side effects by the DMF treatment, which underlines the excellent tolerability of the drug because of high specificity of DMF-induced cell death on activated and malignant T cells. The effects of DMF on tumor growth were, with 30% growth reduction, rather limited, but the effect of DMF on CTCL spreading to distant organs was striking. Nevertheless, to further improve the observed in vivo effects, DMF treatment might be combined with other agents that target NF-κB signaling. Here, bortezomib and KINK-1, an inhibitor of IKKβ, have already shown promising cooperative effects with DMF in vitro.59 Not only NF-κB-targeting agents can be considered, but DMF might also be combined with established CTCL treatments targeting other pathways such as HDAC inhibitors, bexarotene, or interferon to improve therapeutic success and reduce single-agent side effects.
In summary, our data establish DMF as a promising novel therapeutic option in CTCL. DMF restores apoptosis in CTCL by inhibiting constitutively enhanced NF-κB activity of the malignant T cells. Thus, DMF counteracts the major pathogenic factor in CTCL (ie, cell death resistance). Notably, the effects of DMF observed in vitro were comprehensively confirmed in vivo in our xenograft mouse models. DMF-mediated induction of apoptosis in CTCL cells did not only inhibit tumor growth, but also strongly prevented spreading of the tumor from the skin to distant organs. This is highly important because containment of CTCL in the skin is associated with a more favorable prognosis. Because NF-κB activity is selectively enhanced in CTCL cells, DMF treatment leaves other cells and tissues widely unaffected. These features of DMF render it a promising drug with few side effects that may be better tolerated than most established CTCL treatment regimens. Altogether, our findings strongly suggest a prompt evaluation of DMF treatment in CTCL patients in a clinical trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alexandra Démory, Andrea Pohl-Arnold, Daniel Röth, Hiltrud Schönhaber, Brigitte Steinbauer, and Jochen Weber for excellent technical assistance.
This work was supported by the Wilhelm-Sander-Stiftung (project no. 2012.077.1.)
Authorship
Contribution: J.P.N., P.H.K., and K.G. designed the study and planned the experiments. J.P.N., K.M.-D., A.S., M.B., and M.M. performed experiments. J.P.N., K.M.-D., C.G., C.A., S.G., and K.G. evaluated clinical and experimental data. J.P.N., P.H.K., and K.G. prepared and wrote the manuscript.
Conflict-of-interest disclosure: J.P.N. received travel and congress participation funding by TEVA as well as consulting fees by TEVA and Biogen. P.H.K. and K.G. received consulting fees from the Biogen Company. M.B. is an employee of Bayer Pharma AG.
Correspondence: Jan P. Nicolay, MD Division of Immunogenetics (D030), German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; e-mail: j.nicolay@dkfz.de; or Karsten Gülow, Division of Immunogenetics (D030), German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; e-mail: k.guelow@dkfz.de.

![Figure 2. DMF inhibits NF-κB activity in CTCL cells. (A) Specific luminescence of the NF-κB luciferase assay in J16 and HH cells 24 hours after transfection without treatment (n = 3, each). (B) Specific luminescence of the NF-κB luciferase assay in J16 and HH cells 24 hours after transfection with and without treatment with 50 µM DMF for 24 hours. The specific NF-κB activity of untreated J16 cells was normalized to 1 and all other activities were normalized to that value (n = 3 each). (C) Relative IκBα expression measured by qRT-PCR in SeAx (upper) and HH (lower) cells 1 to 3 hours after treatment with 50 µM of either DMF or MMF, normalized to GAPDH (n = 3 each). (D) TNF-α concentration in supernatants of HH cells either untreated or treated with 50 µM DMF for 12 hours (n = 3 each). (E) Normalized nuclear p65 activity measured by binding ELISA in HH and SeAx cells upon treatment with different concentrations of DMF and MMF for 1 hour (upper) and 16 hours (lower), (n = 3 each). DMSO, dimethyl sulfoxide. (F) Relative changes in expression levels of NF-κB target genes measured by qRT-PCR array after 3 hours of treatment with DMF and MMF (30 µM each). The quantitative values are (expression [DMF treatment])/(expression(MMF treatment)). (n = 3, each) *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/6/10.1182_blood-2016-01-694117/4/m_805f2.jpeg?Expires=1769115047&Signature=L1Zgb5ByODCoxLKsOu2LWoPe2ZdOVkY9OhjCU~mXilW~vhvXKlNx3qAD3SdUL9IAt3JANUAgvJPHgH6eUzM62q8I44~Fh1wJTF61jjZC8f0~JMLlBHEXj5IM1863074IN1sCxShZVwjyA14i8TC6DeIcS0QCP6V4sh1out7LOM4VQm64mjeYF35MmYhWAipZYwf~Hfi7sRlexR3tZp98Ud84pv0IvUx0bg-siBRYOvVtELQD0JPv527M7ftmTEVqd84htXmPQBhIJ5mJ0oIqwq66ryv1QU9J2bfR8FXy3hzyqlXMOhzvmE8PO7O6DRpZmy5IGgOFZHAAA1HFHo9bzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)