Key Points
TPO-RAs shift monocyte FcγR balance toward the inhibitory FcγRIIb and correct the enhanced phagocytic capacity of macrophages in ITP.
Abstract
Elevated expression of the activating Fcγ receptor (FcγR) I and FcγRIIa together with decreased expression of the inhibitory FcγRIIb are involved in the pathogenesis of primary immune thrombocytopenia (ITP). Thrombopoietin receptor agonists (TPO-RAs) have been used clinically for the management of ITP; however, little is known about the effect of TPO-RAs on FcγR modulation in ITP. In this prospective study, we measured the alteration in monocyte FcγR expression from 21 corticosteroid-resistant/relapsed patients with chronic ITP receiving eltrombopag therapy. Results showed that the mRNA and protein levels of FcγRIIb were significantly elevated after 6-week eltrombopag treatment. Concurrently, FcγRI and IIa levels decreased remarkably, whereas FcγRIII expression did not change. In vitro phagocytosis assays indicated that a shift in the balance of FcγR toward inhibitory FcγRIIb on monocytes was accompanied with a considerable decrease in monocyte/macrophage phagocytic capacity. The response to eltrombopag therapy in patients with ITP was associated with FcγR phenotype and functional changes of monocytes/macrophages. Moreover, the plasma transforming growth factor-β1 (TGF-β1) concentrations increased significantly in eltrombopag responders. Modulation of monocyte FcγR balance by TPO-RAs was also found in a murine model of ITP established by transferring splenocytes from immunized CD61 knockout mice into CD61+ severe combined immunodeficient mice. Romiplostim administration in ITP mice significantly upregulated inhibitory FcγRII expression and downregulated activating FcγRI expression. These findings showed that recovery of platelet counts after TPO-RA treatment in ITP is associated with the restoration of FcγR balance toward the inhibitory FcγRIIb on monocytes, and suggested that thrombopoietic agents have a profound effect on immune modulation in ITP. This study is registered at ClinicalTrials.gov as #NCT01864512.
Introduction
Fcγ receptors (FcγRs) are a heterogeneous group of cell-surface glycoproteins that provide important links between humoral and cellular immunity. Three classes of FcγRs have been identified on human leukocytes: FcγRI, II, and III, with the isoforms IIa and IIb, and IIIa and IIIb. Functionally, FcγRs can be divided into the activating receptors (FcγRI, IIa, and III) and the inhibitory receptor (FcγRIIb). Ligation of FcγRI, IIa, and III activates inflammatory cells, such as monocytes/macrophages or neutrophils, through an intracellular immunoreceptor tyrosine-based activation motif (ITAM), whereas crosslinking of FcγRIIb inhibits functions of the activating FcγRs via an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM).1,2 The balance of the activating and inhibitory FcγRs determines the magnitude of the cellular response. A growing body of evidence has convincingly shown that the disturbed balance of the activating and inhibitory FcγRs plays an important role in the pathophysiology of certain autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus.3,4 Moreover, a therapeutic response in RA and systemic lupus erythematosus is often associated with restoration of the balance between activating and inhibitory FcγRs.5,6
Primary immune thrombocytopenia (ITP), one of the most common bleeding disorders, is an acquired autoimmune disease. Autoantibody-mediated platelet clearance in the reticuloendothelial system (RES) is the traditionally identified mechanism for platelet destruction in ITP.7,8 Monocytes/macrophages have been implicated as key players in the perturbation of immune tolerance in ITP.9-12 Because autoantibodies targeting platelet glycoproteins (GPs) in ITP are predominantly of the IgG1 subclass, antibody-coated platelets are prone to phagocytic removal via the FcγR-bearing macrophages.13 Moreover, ligation of FcγRs on monocytes/macrophages by GP-specific autoantibodies initiates antigen presentation and proinflammatory cytokine production.14,15 Genotype analysis conducted in patients with ITP indicated that polymorphisms of FcγRIIb-232I/T or FcγRIIIa-158V/F were correlated with disease susceptibility or therapeutic response.16 Samuelsson et al showed that the therapeutic effect of intravenous immunoglobulin (IVIg) in a murine model of ITP was associated with induction of inhibitory FcγRIIb on splenic macrophages.17 In addition, our group also observed a significant decrease in the inhibitory FcγRIIb expression that paralleled with a remarkable increase in activating FcγRI and FcγRIIa expression in active patients with ITP compared with healthy controls.14 Treatment regimens, such as high-dose dexamethasone or Helicobacter pylori (H pylori) eradication, could shift monocyte FcγR balance toward the inhibitory FcγRIIb in treatment-sensitive patients.18,19 Further investigation into FcγR regulation might provide new insights into the mechanisms of immunomodulation therapies in ITP.
Established therapies for ITP include glucocorticosteroids (GCs), IVIg, splenectomy, rituximab, and other immunosuppressive drugs. In response to findings that identified decreased platelet production in ITP caused by relatively insufficient levels of thrombopoietin (TPO), a number of thrombopoietic agents have been developed. Eltrombopag and romiplostim, 2 types of TPO receptor agonists (TPO-RAs), have been shown to be effective for the management of ITP.20,21 In addition to their role in stimulating platelet production from megakaryocytes, TPO-RAs have an additional effect on immunoregulation. Bao et al recently observed that improved activity of regulatory T cells (Tregs) coincided with a remarkable decrease in effector T helper–cell function in TPO-RA–treated patients with ITP.22 Similar results were shown in a murine model of ITP that short-term TPO-RA treatment could promote the peripheral induction of Tregs and suppress T-cell responses to platelet autoantigens.23 In addition, thrombopoietic treatment could rectify the defects of regulatory B cells (Bregs) in immune regulation in patients with chronic ITP.24 However, the effects of TPO-RAs on FcγR modulation remain unknown in ITP. In this prospective study, we demonstrated that eltrombopag treatment increased the expression of FcγRIIb and decreased the expression of FcγRIIa and FcγRI on monocytes from patients with ITP. Furthermore, romiplostim administration significantly upregulated inhibitory FcγRII expression and downregulated activating FcγRI expression in ITP mice, suggesting that TPO-RAs could attenuate FcγR-mediated monocyte activation by shifting the FcγR balance toward the inhibitory FcγRIIb. These findings offer a potential mechanism of TPO-RAs in the management of chronic ITP.
Materials and methods
Patients
Patients were enrolled between June 2013 and April 2014 at the Department of Hematology, Qilu Hospital, Shandong University, Jinan, and at the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China. ITP was diagnosed according to the practice guidelines of the International Working Group on ITP.25 Participants were required to be ≥18 years, have at least a 12-month history of ITP, a pretreatment platelet count <30 × 109/L, and have relapsed ITP or be unresponsive to glucocorticosteroid therapy. Previous therapy, including rescue, must have been completed at least 6 weeks before enrollment.
Patients with any of the following were excluded: (1) a history of thrombopoietic agent use or thrombosis; (2) liver, kidney, cardiac, or pulmonary dysfunction; (3) a clinical history of hepatitis B/C virus or human immunodeficiency virus infection; or (4) female subjects who were pregnant or nursing. The study protocol was approved by the Medical Ethical Committees of each institution. Informed consent was obtained from all patients before enrollment in accordance with the Declaration of Helsinki. This study is registered with ClinicalTrials.gov (#NCT01864512).
Study design
This prospective cohort study aimed to determine the efficacy of eltrombopag for the management of chronic ITP and to investigate the alteration of FcγR phenotype on monocytes after eltrombopag administration. Eltrombopag (GlaxoSmithKline, Ware, UK) was administered orally with an initial dose of 25 mg once daily. The dose could be increased to 50 or 75 mg once daily to maintain platelet counts ≥50 × 109/L. To reduce the risk of thrombocytosis, treatment was discontinued when platelet counts exceeded 250 × 109/L.
The primary end points were the alteration of the FcγR phenotype on monocytes (including FcγRI, II, and III) and phagocytic ability 6 weeks after initiation of treatment. Secondary end points included complete response (CR), response (R), no response (NR), time to response (TTR), and adverse events (AEs) during the 6 weeks of treatment. CR was defined as platelet count ≥100 × 109/L and absence of bleeding; R as platelet count range 30 to 100 × 109/L and at least a twofold increase of the baseline count without bleeding; NR as platelet count <30 × 109/L or less than doubling of the baseline platelet count or bleeding; and TTR as the time from baseline to response. Platelet counts should be confirmed on 2 separate occasions at least 7 days apart when defining CR or R. Patients who withdrew prematurely because their platelet count surpassed 250 × 109 /L were considered responders. Patients who discontinued treatment of any other reasons (eg, AEs, lack of efficacy) were considered nonresponders. AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs (version 3.0).25 All patients were assessed weekly for efficacy, safety, and tolerability of the treatment during the 6-week treatment period.
Flow cytometry and real-time RT-PCR analysis of monocyte FcγRs
Surface expression of FcγRI, FcγRII, FcγRIII, and FcγRIIb on circulating monocytes was determined by flow cytometry. mRNA expression of FcγRIIa and FcγRIIb on monocytes was measured by real-time reverse transcription polymerase chain reaction (RT-PCR). The reagents and experimental protocols are described in detail in the supplemental Methods, available on the Blood Web site. Primer sequences for human FcγRIIa, FcγRIIb, and glyceraldehyde-3-phosphate dehydrogenase as well as murine FcγRII, FcγRIII, and β-actin, and PCR cycling conditions are listed in Table 1.
Primers and conditions for real-time PCR
| Gene . | Primer sequence (5′-3′) . | Annealing temperature (°C) . | Product length (bp) . | |
|---|---|---|---|---|
| Forward . | Reverse . | |||
| Human FcγRIIa | ATCATTGTGGCTGTGGTCATTG | TGTTTCATAGTCATTGTTGGTTTCTTC | 65 | 160 |
| Human FcγRIIb | ATTCCTGGCTCCTGTTGCTG | GAATGGAGTCGCTCTCAGGG | 65 | 144 |
| Human GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA | 65 | 138 |
| Mouse FcγRIIb | CAGAATGCACACTCTGGAAGC | GGGTCCCTTCGCACATCAG | 60 | 169 |
| Mouse FcγRIII | AGGGCCTCCATCTGGACTG | GTGGTTCTGGTAATCATGCTCTG | 60 | 160 |
| Mouse β-actin | CGGTTCCGATGCCCTGAGGCTCTT | CGTCACACTTCATGATGGAATTGA | 60 | 100 |
| Gene . | Primer sequence (5′-3′) . | Annealing temperature (°C) . | Product length (bp) . | |
|---|---|---|---|---|
| Forward . | Reverse . | |||
| Human FcγRIIa | ATCATTGTGGCTGTGGTCATTG | TGTTTCATAGTCATTGTTGGTTTCTTC | 65 | 160 |
| Human FcγRIIb | ATTCCTGGCTCCTGTTGCTG | GAATGGAGTCGCTCTCAGGG | 65 | 144 |
| Human GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA | 65 | 138 |
| Mouse FcγRIIb | CAGAATGCACACTCTGGAAGC | GGGTCCCTTCGCACATCAG | 60 | 169 |
| Mouse FcγRIII | AGGGCCTCCATCTGGACTG | GTGGTTCTGGTAATCATGCTCTG | 60 | 160 |
| Mouse β-actin | CGGTTCCGATGCCCTGAGGCTCTT | CGTCACACTTCATGATGGAATTGA | 60 | 100 |
In vitro phagocytosis assays
Platelet 5-chloromethylfluorescein diacetate–labeling and opsonization, macrophage preparation, and in vitro phagocytosis of opsonized platelet were carried out according to a previously described method with a few modifications.26,27 FcγR profile on monocyte-derived macrophages in 6 additionally recruited patients with ITP was determined, and an activating FcγR phenotype was found compared with healthy controls (supplemental Figure 1). Protocols are given in detail in the supplemental Methods.
TGF-β1 enzyme-linked immunosorbent assay
The levels of plasma transforming growth factor-β1 (TGF-β1) were determined by an enzyme-linked immunosorbent assay (R&D Systems) following the manufacturer’s recommendations. The lower detection limit for TGF-β1 was 15.4 pg/mL.
Determination of FcγR protein expression on monocytes/macrophages from ITP mice
The active ITP murine model was constructed according to a previously established method.28 Details of ITP mouse generation and romiplostim administration are presented in the supplemental data. Spleens from ITP mice were isolated and homogenized. Splenocytes (1 × 106) were stained with fluorescein isothiocyanate–conjugated anti-mouse-CD11b mAbs (Clone M1/70), in combination with phycoerythrin (PE)-conjugated anti-mouse-CD64 (FcγRI) (clone X54-5/7.1) and allophycocyanin-conjugated anti-mouse-CD16/32 (FcγRIII/FcγRII) (clone 93) for 30 minutes in the dark. Erythrocytes were lysed by ACK lysing solution. Cells were washed in phosphate-buffered saline/1% bovine serum albumin/0.01% NaN3 and fixed in 1% paraformaldehyde phosphate-buffered saline. Analysis was performed with a Beckman Gallios Flow Cytometer (Beckman Coulter) with Gallios Cytometry List Mode Data Acquisition & Analysis Software (Beckman Coulter). The gating strategy of SSClowCD11bhigh for macrophage identification has been used in the present study, which could distinguish macrophages from neutrophils and lymphocytes.29-31 PE conjugated anti-CD49b (clone DX5), allophycocyanin-conjugated anti-CD11c (clone N418), and PE-Cy7–conjugated anti-F4/80 (clone BM8) were used to analyze the proportion of natural killer cells, dendritic cells, and macrophages. FcγRI and FcγRII/III expression on CD11b+-gated resident macrophages were presented as mean fluorescence intensity (MFI) and were calculated based on the intensity of the cells incubated with appropriate isotype-matched control IgG as a reference.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software. All continuous values were expressed as means ± standard deviation. Descriptive statistics were used to summarize demographic and baseline clinical characteristics and safety data. The statistical significance between 2 groups was determined by Fisher’s exact test or Mann-Whitney U test, as appropriate. Changes in the absolute values at different time points from the baseline value at week 0 were compared by paired Student t test. Nonparametric Spearman correlation was used to analyze the expression of FcγRs and platelet counts. P < .05 was considered statistically significant.
Results
Patient demographics and disposition
A total of 21 corticosteroid-resistant or -relapsed patients with ITP (12 females, 9 males) were enrolled in the study and received eltrombopag treatment. The median age of patients was 51 years (range 21-84). The duration of ITP from the time of diagnosis ranged from 13 months to 433 months, with the median course of 39 months. The most commonly used previously therapy was high-dose dexamethasone (HD-DXM) (85.71%), and the second most commonly used was prednisone (80.95%). Seven patients (33.33%) had received IVIg treatment, and 6 (28.57%) had received vincristine (VCR) before the study entry. The baseline platelet counts ranged between 4 and 25 × 109/L, with a median count of 13 × 109 /L (Table 2).
Demographic and clinical characteristics of patients with chronic ITP treated with eltrombopag
| Patient No. . | Sex/Age (y) . | Disease duration (y) . | Previous treatment . | Platelet count (×109/L) . | |
|---|---|---|---|---|---|
| Pretreatment . | Posttreatment . | ||||
| 1 | M/25 | 2+ | Steroid, VCR | 13 | 112 |
| 2 | M/24 | 4+ | Steroids | 12 | 5 |
| 3 | M/51 | 2+ | Steroids, IVIg | 8 | 25 |
| 4 | F/35 | 7+ | Steroids | 18 | 22 |
| 5 | F/52 | 4+ | Steroids, VCR | 17 | 105 |
| 6 | M/48 | 2– | Steroids, VCR | 4 | 167 |
| 7 | F/63 | 2+ | Steroids | 12 | 15 |
| 8 | F/24 | 1+ | Steroids; IVIg | 8 | 50 |
| 9 | F/60 | 10+ | Steroids, herbs | 21 | 71 |
| 10 | F/62 | 2+ | Steroids | 9 | 124 |
| 11 | M/55 | 30+ | Steroids | 21 | 59 |
| 12 | M/54 | 3+ | Steroids | 19 | 247 |
| 13 | M/84 | 2+ | Steroids, IVIg | 7 | 37 |
| 14 | F/58 | 4+ | Steroids, herbs | 19 | 51 |
| 15 | M/59 | 3+ | Steroids, IVIg, VCR | 13 | 15 |
| 16 | F/50 | 11+ | Steroids, herbs | 21 | 55 |
| 17 | M/27 | 1+ | Steroids | 25 | 62 |
| 18 | F/54 | 7+ | Steroids, IVIg | 22 | 69 |
| 19 | F/42 | 1+ | Steroids | 17 | 57 |
| 20 | F/44 | 2+ | Steroids, IVIg, VCR | 6 | 104 |
| 21 | F/21 | 7+ | Steroids, IVIg, VCR | 4 | 33* |
| Patient No. . | Sex/Age (y) . | Disease duration (y) . | Previous treatment . | Platelet count (×109/L) . | |
|---|---|---|---|---|---|
| Pretreatment . | Posttreatment . | ||||
| 1 | M/25 | 2+ | Steroid, VCR | 13 | 112 |
| 2 | M/24 | 4+ | Steroids | 12 | 5 |
| 3 | M/51 | 2+ | Steroids, IVIg | 8 | 25 |
| 4 | F/35 | 7+ | Steroids | 18 | 22 |
| 5 | F/52 | 4+ | Steroids, VCR | 17 | 105 |
| 6 | M/48 | 2– | Steroids, VCR | 4 | 167 |
| 7 | F/63 | 2+ | Steroids | 12 | 15 |
| 8 | F/24 | 1+ | Steroids; IVIg | 8 | 50 |
| 9 | F/60 | 10+ | Steroids, herbs | 21 | 71 |
| 10 | F/62 | 2+ | Steroids | 9 | 124 |
| 11 | M/55 | 30+ | Steroids | 21 | 59 |
| 12 | M/54 | 3+ | Steroids | 19 | 247 |
| 13 | M/84 | 2+ | Steroids, IVIg | 7 | 37 |
| 14 | F/58 | 4+ | Steroids, herbs | 19 | 51 |
| 15 | M/59 | 3+ | Steroids, IVIg, VCR | 13 | 15 |
| 16 | F/50 | 11+ | Steroids, herbs | 21 | 55 |
| 17 | M/27 | 1+ | Steroids | 25 | 62 |
| 18 | F/54 | 7+ | Steroids, IVIg | 22 | 69 |
| 19 | F/42 | 1+ | Steroids | 17 | 57 |
| 20 | F/44 | 2+ | Steroids, IVIg, VCR | 6 | 104 |
| 21 | F/21 | 7+ | Steroids, IVIg, VCR | 4 | 33* |
IVIg, intravenous immunoglobulin; VCR, vincristine.
+, more than; –,less than.
Patients whose platelets were ≥30 × 109/L but showed petechial bleeding after therapy; steroids included high-dose dexamethasone (HD-DXM), prednisone, or methylprednisolone.
Efficacy and safety of eltrombopag
Platelet counts at baseline and 6 weeks after initiation of eltrombopag administration are shown in Table 2. Response was achieved in 15 (71.43%) patients, including 6 with CR (28.57%), and nonresponse was shown in 6 (28.57%) of the 21 patients. Two patients received platelet transfusion during the study. The median daily dose of eltrombopag was 50 mg, which was stable throughout the study. Most of the patients showed good tolerability to eltrombopag therapy. Consistent with previous reports,32-34 the most common adverse reactions were headache (4 patients), fatigue (4 patients), diarrhea (1 patient), or transient liver transaminase increase (3 patients). One patient suffered from intussusception after a week of eltrombopag administration, which was proved irrelevant to the medication. After surgical treatment, the patient continued eltrombopag therapy and responded during the 6-week observation period. During the 4-month follow-up, one patient of the total 15 responders (6.7%) relapsed at 15 weeks after treatment had ended.
Effects of eltrombopag on FcγRI, FcγRII, and FcγRIII expression on circulating monocytes
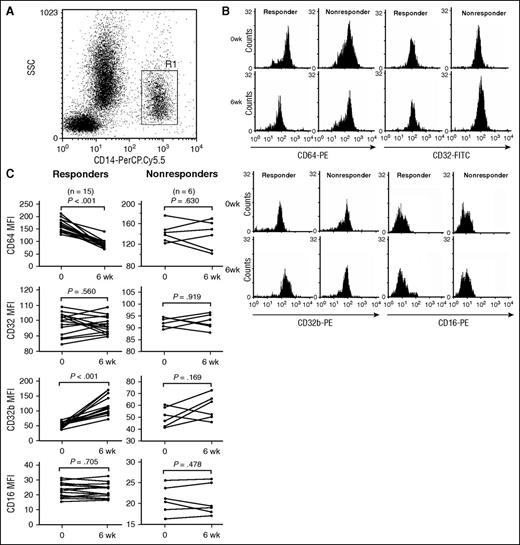
Changes in the expression of FcγRs on circulating monocytes from patients with ITP were determined by flow cytometry. Levels of FcγRI on monocytes decreased considerably after 6 weeks of eltrombopag administration (155.4 ± 25.16 vs 99.88 ± 31.55; P < .001). The inhibitory FcγRIIb expression on monocytes showed a significant increase after eltrombopag treatment (50.86 ± 8.48 vs 97.53 ± 36.28; P < .001), whereas no significant changes were observed in total FcγRII (IIa + IIb) expression on monocytes (96.32 ± 6.65 vs 95.64 ± 5.37; P = .597) or in FcγRIII expression after eltrombopag treatment (22.13 ± 3.34 vs 22.1 ± 3.19; P = .952).
The association between response to eltrombopag therapy and the FcγR phenotype on monocytes was also analyzed. As is shown in Figure 1C, in eltrombopag-responsive patients, levels of FcγRIIb on monocytes increased significantly compared with those before treatment (51.0 ± 8.88 vs 113.06 ± 30.62; P < .001), whereas levels of FcγRI on monocytes decreased remarkably after eltrombopag therapy (161.03 ± 25.62 vs 85.15 ± 18.22; P < .001). By contrast, in eltrombopag-nonresponsive patients, eltrombopag administration did not significantly affect monocyte expression of FcγRI (141.33 ± 19 vs 136.73 ± 27.77; P = .63), FcγRII (92.39 ± 1.99 vs 92.54 ± 2.98; P = .919), or FcγRIIb (50.53 ± 8.14 vs 58.71 ± 10.36; P = .169). Furthermore, monocytic FcγRIII expression in neither responders nor nonresponders showed statistical significance after eltrombopag treatment (responders: 22.5 ± 3.61 vs 22.69 ± 3.32, P = .705; nonresponders: 21.2 ± 2.56 vs 20.63 ± 2.50, P = .478).
FcγR expression on monocytes from patients with ITP before or after eltrombopag treatment. (A) Representative scattergrams of surface expression of CD14+ monocytes from a patient with ITP. R1 represented CD14+ monocytes. (B) Representative histogram of MFI of FcγRI/CD64, FcγRII/CD32, FcγRIIb/CD32b, and FcγRIII/CD16 from a responder and a nonresponder before and after eltrombopag therapy. (C) MFI of FcγRI/CD64, FcγRII/CD32, FcγRIIb/CD32b, and FcγRIII/CD16 on monocytes before treatment and 6 weeks after eltrombopag treatment in 15 ITP responders and 6 responders. Differences before and after treatment were determined by paired Student t tests.
FcγR expression on monocytes from patients with ITP before or after eltrombopag treatment. (A) Representative scattergrams of surface expression of CD14+ monocytes from a patient with ITP. R1 represented CD14+ monocytes. (B) Representative histogram of MFI of FcγRI/CD64, FcγRII/CD32, FcγRIIb/CD32b, and FcγRIII/CD16 from a responder and a nonresponder before and after eltrombopag therapy. (C) MFI of FcγRI/CD64, FcγRII/CD32, FcγRIIb/CD32b, and FcγRIII/CD16 on monocytes before treatment and 6 weeks after eltrombopag treatment in 15 ITP responders and 6 responders. Differences before and after treatment were determined by paired Student t tests.
FcγRIIa and IIb gene expression in monocytes
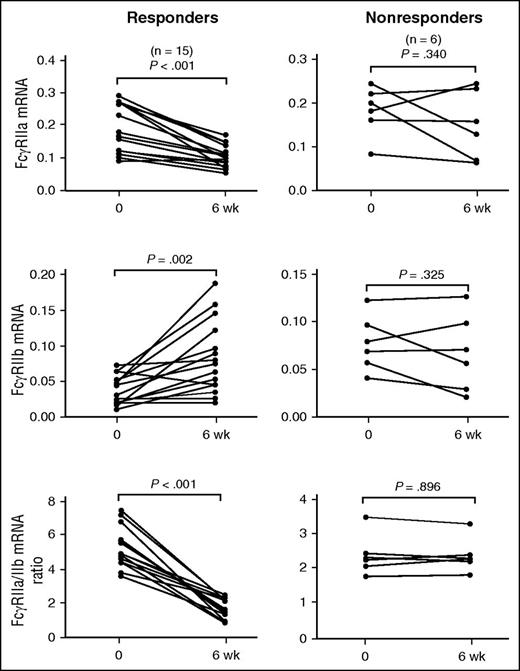
Real-time RT-PCR was performed on sorted monocytes from patients before and after eltrombopag treatment. Results revealed that the ratio of FcγRIIa/IIb mRNA expression in monocytes decreased significantly after 6 weeks of eltrombopag treatment (4.38 ± 1.64 vs 1.76 ± 0.7; P < .001). In eltrombopag-responsive patients, significantly decreased FcγRIIa/IIb mRNA ratio was observed 6 weeks after initiation of eltrombopag administration (5.16 ± 1.2 vs 1.5 ± 0.59; P < .001). However, this downregulation of the FcγRIIa/IIb mRNA ratio was not observed in eltrombopag-nonresponsive patients (2.43 ± 0.61 vs 2.42 ± 0.51; P = .896). Consistent with the changes at protein levels, monocytic FcγRIIb mRNA expression was significantly elevated in eltrombopag-responsive patients (0.0387 ± 0.02 vs 0.0838 ± 0.05; P = .002), whereas upregulation of FcγRIIb mRNA was not observed in eltrombopag-nonresponsive patients (0.0798 ± 0.0302 vs 0.069 ± 0.0419; P = .325; Figure 2). These findings suggested that recovery of platelet counts by eltrombopag was associated with the restoration of an inhibitory FcγR phenotype on monocytes in patients with ITP.
FcγRIIa and FcγRIIb mRNA levels in monocytes from patients with ITP before and after treatment. The mRNA levels of FcγRIIa and FcγRIIb, and the FcγRIIa/IIb mRNA ratio on monocytes before treatment and 6 weeks after eltrombopag therapy in 15 ITP responders and 6 nonresponders. Differences before and after treatment were determined by paired Student t tests.
FcγRIIa and FcγRIIb mRNA levels in monocytes from patients with ITP before and after treatment. The mRNA levels of FcγRIIa and FcγRIIb, and the FcγRIIa/IIb mRNA ratio on monocytes before treatment and 6 weeks after eltrombopag therapy in 15 ITP responders and 6 nonresponders. Differences before and after treatment were determined by paired Student t tests.
Phagocytic capacity of monocytes/macrophages after eltrombopag treatment in patients with ITP
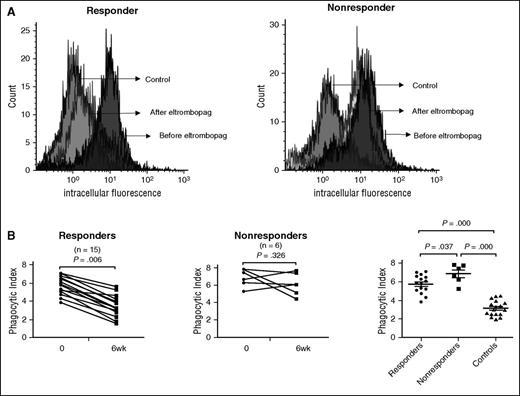
To evaluate whether shift in the FcγR status of circulating monocytes was associated with functional changes, monocyte-derived macrophages from patients with ITP before and after eltrombopag therapy were incubated with opsonized 5-chloromethylfluorescein diacetate–labeled platelets for phagocytosis assays. As expected, in monocyte-derived macrophages from eltrombopag responders, a shift toward the inhibitory FcγRIIb was accompanied by significantly decreased phagocytic capacity after 6 weeks of eltrombopag treatment (5.77 ± 0.10 vs 3.54 ± 1.17; P = .006; Figure 3B). No statistically significant changes were found in the phagocytic capacity of macrophages from eltrombopag nonresponders (6.88 ± 1.01 vs 6.11 ± 1.26; P = .326; Figure 3B). Furthermore, macrophages from eltrombopag nonresponders demonstrated significantly higher phagocytic capacity compared with those from eltrombopag responders (5.77 ± 0.10 vs 6.88 ± 1.01; P = .037; Figure 3B).
Phagocytic capacity of monocyte-derived macrophages before and after eltrombopag treatment in patients with ITP. (A) Representative histogram of intracellular fluorescence from 1 responder and 1 nonresponder before and after eltrombopag therapy. (B) Phagocytosis index of monocyte-derived macrophages before treatment and 6 weeks after eltrombopag treatment in 15 ITP responders and 6 nonresponders. Differences before and after treatment were determined by paired Student t tests. Differences between responders, nonresponders, and controls were determined by Mann-Whitney U tests.
Phagocytic capacity of monocyte-derived macrophages before and after eltrombopag treatment in patients with ITP. (A) Representative histogram of intracellular fluorescence from 1 responder and 1 nonresponder before and after eltrombopag therapy. (B) Phagocytosis index of monocyte-derived macrophages before treatment and 6 weeks after eltrombopag treatment in 15 ITP responders and 6 nonresponders. Differences before and after treatment were determined by paired Student t tests. Differences between responders, nonresponders, and controls were determined by Mann-Whitney U tests.
Plasma TGF-β1 levels after eltrombopag treatment in patients with ITP
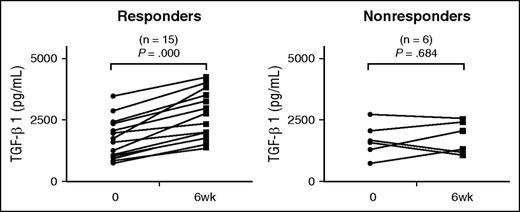
TGF-β1 is an antiinflammatory cytokine, which can downregulate activating FcγRs and enhance expression of the inhibitory receptor, FcγRIIb. To evaluate whether TGF-β1 was involved in TPO-RA–mediated FcγR balance, we measured plasma TGF-β1 in patients with ITP after eltrombopag treatment. As shown in Figure 4, plasma TGF-β1 concentrations significantly increased in eltrombopag responders (1697.67 ± 814.42 vs 2624.80 ± 959.29 pg/mL; P < .001). No significant changes were observed in eltrombopag nonresponders (1691.65 ± 674.48 vs 1789.17 ± 661.98 pg/mL; P = .684).
Plasma TGF-β1 levels before and after eltrombopag treatment in patients with ITP. Plasma TGF-β1 before and 6 weeks after eltrombopag treatment in 15 ITP responders and 6 nonresponders. Differences were determined by paired Student t tests.
Plasma TGF-β1 levels before and after eltrombopag treatment in patients with ITP. Plasma TGF-β1 before and 6 weeks after eltrombopag treatment in 15 ITP responders and 6 nonresponders. Differences were determined by paired Student t tests.
In vivo regulation of FcγR profile on monocytes/macrophages by romiplostim in ITP mice
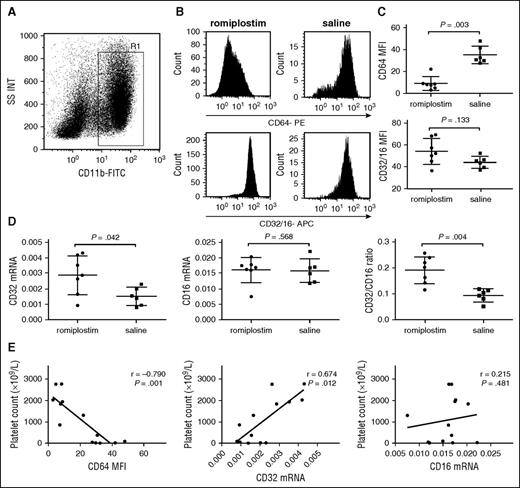
It is well known that monocytes/macrophages from mice only express one FcγRII, which corresponds with human inhibitory FcγRIIb. To investigate the effect of romiplostim on the expression of FcγRs on resident macrophages of ITP mice, protein and mRNA expression levels of FcγRI, FcγRII, and FcγRIII were measured by flow cytometry and real-time RT-PCR, respectively. As described in the supplemental data, the fraction of F4/80+ macrophages in SSClowCD11bhigh population was >90% in spleen of ITP mice (supplemental Figure 2). Romiplostim administration downregulated surface FcγRI levels significantly on splenic macrophages compared with that of controls (8.90 ± 6.16 vs 35.13 ± 8.03, P = .003; Figure 5C). There was a nonsignificant increase in FcγRII/III expression (54.65 ± 12.07 vs 44.42 ± 5.56, P = .133; Figure 5C). To distinguish FcγRII from FcγRIII expression in macrophages from ITP mice, real-time RT-PCR was performed. Results showed that the ratio of FcγRII/III mRNA expression increased significantly after romiplostim injection compared with controls (0.191 ± 0.053 vs 0.094 ± 0.024; P = .004; Figure 5D). Romiplastim administration upregulated FcγRII mRNA expression significantly on splenic macrophages, whereas no obvious effect on FcγR III was found (FcγRII: 0.0029 ± 0.0013 vs 0.0015 ± 0.0006, P = .042; FcγRIII: 0.016 ± 0.004 vs 0.0158 ± 0.0038, P = .568; Figure 5D). Moreover, the level of FcγRI negatively correlated with platelet counts(R = −0.790, P = .001), whereas the level of FcγRII positively correlated with platelet counts (R = 0.674, P = .012 ; Figure 5E). To ensure the immune system of ITP mice did not react against the human romiplostim, anti-TPO antibodies were also determined using an enzyme-linked immunosorbent assay kit. We did not observe any endogenous anti-TPO antibodies in the ITP mouse serum (supplemental data).
In vivo regulation of FcγR profile on monocytes/macrophages by romiplostim in ITP mice. (A) Representative scattergrams of surface expression of CD11b+ splenic macrophages from an ITP mouse. R1 represented CD11b+ macrophages. (B) Representative histogram of mean fluorescence intensity (MFI) of FcγRI/CD64 and FcγRII/III/CD32/16 of splenic macrophages from 2 mice with romiplostim therapy and saline, respectively. (C) MFI of FcγRI/CD64 and FcγRII/III/CD32/16 on splenic macrophages with eltrombopag treatment or saline in ITP mice. (D) The mRNA levels of FcγRII/CD32 and FcγRIII/CD16 on splenic macrophages with eltrombopag treatment and saline in ITP mice. (E) Correlative analysis of the expressions of FcγRs and platelet counts. The differences between the 2 groups were determined by Mann-Whitney U tests. Correlative analysis was determined by nonparametric Spearman correlation.
In vivo regulation of FcγR profile on monocytes/macrophages by romiplostim in ITP mice. (A) Representative scattergrams of surface expression of CD11b+ splenic macrophages from an ITP mouse. R1 represented CD11b+ macrophages. (B) Representative histogram of mean fluorescence intensity (MFI) of FcγRI/CD64 and FcγRII/III/CD32/16 of splenic macrophages from 2 mice with romiplostim therapy and saline, respectively. (C) MFI of FcγRI/CD64 and FcγRII/III/CD32/16 on splenic macrophages with eltrombopag treatment or saline in ITP mice. (D) The mRNA levels of FcγRII/CD32 and FcγRIII/CD16 on splenic macrophages with eltrombopag treatment and saline in ITP mice. (E) Correlative analysis of the expressions of FcγRs and platelet counts. The differences between the 2 groups were determined by Mann-Whitney U tests. Correlative analysis was determined by nonparametric Spearman correlation.
Discussion
In this prospective cohort study, eltrombopag was administered at doses of 25 to 75 mg once daily in corticosteroid-resistant or -relapsed patients with chronic ITP. After 6-week eltrombopag administration, 71.43% of patients achieved response. These results were consistent with previous studies that showed that eltrombopag was effective in 70% of patients with chronic ITP.32 In the present study, an extensive phenotypic analysis of circulating monocytes was carried out by flow cytometry before and after 6 weeks of eltrombopag treatment. It was demonstrated that eltrombopag therapy downregulated the activating FcγRI expression and upregulated the inhibitory FcγRIIb expression on circulating monocytes in eltrombopag responders. Real-time RT-PCR analysis further revealed an elevation of the FcγRIIb mRNA expression together with a decrease of FcγRIIa mRNA expression after eltrombopag treatment in responders. By contrast, neither the protein levels of FcγRI and FcγRIIb nor the mNRA levels of FcγRIIa and FcγRIIb were significantly affected by eltrombopag therapy in eltrombopag nonresponders.
We did not observe any statistical change in monocyte FcγRIII expression either in responders or in nonresponders after TPO-RA treatment, which was slightly different from previous reports.10 In their work, Zhong and colleagues found that ITP nonresponders had increased CD16+ monocyte frequencies compared with responders or healthy controls. However, the authors did not observe any statistical significance in frequency of circulating CD14+CD16– monocytes between responders and nonresponders after TPO-RA treatment.10 It is well known that the CD14dimCD16+ subset only accounted for a small proportion of the total monocyte population according to the existing literature.29,35-37 These might explain why no statistical change in surface levels of CD16 on total monocyte population after TPO-RA treatment in responders and nonresponders in our present study.
To further investigate the role of TPO-RAs in modulation of FcγRs on macrophages from the RES rather than circulating monocytes, FcγR phenotypes of splenic macrophages from ITP mice were evaluated. Because eltrombopag could not bind murine TPO-R, romiplostim, an Fc-peptide fusion protein that bind to TPO-R of various species, was used to stimulate platelet production in the murine model of ITP. After 2 weeks of romiplostim injection, platelet counts in ITP mice elevated remarkably, and a significant increase in inhibitory FcγRII paralleled a decrease in activating FcγRI on splenic macrophages.
It has been well established that the functional properties of monocytes or macrophages, such as abilities of phagocytosis and antigen presentation, are associated with the balance of the activating and inhibitory FcγRs.38-40 Our study showed that the FcγR-mediated phagocytic capacity of monocyte-derived macrophages from patients with ITP decreased substantially in eltrombopag responders, which was consistent with the changing trend of FcγRs toward an inhibitory phenotype. In addition, platelet destruction in spleen of ITP murine model was attenuated after TPO-RA administration as described in supplemental data, which indicated that TPO-RAs could lower platelet destruction aside from stimulating platelet production. Restoration of FcγR balance and enhancement of Treg or Breg suppressive capacity after TPO-RA therapy might exert crucial roles in decreasing platelet destruction in ITP.23,24 The molecular basis for the changes in monocyte phenotype remains unknown. Given that immune modulation by Th1/Th2-related cytokines has been proposed to be associated with FcγR regulation, we also measured plasma IFN-γ and IL-4 before and after eltrombopag therapy in the present study. However, we did not observe any significant changes in plasma levels of IFN-γ or IL-4 after 6 weeks of eltrombopag administration in either responders or nonresponders (data not shown), suggesting that modulation of monocyte FcγRs might not be related to Th profile change.
Eltrombopag is a class of orally bioavailable, small-molecule, nonpeptide TPO-RA approved for the management of patients with chronic ITP in whom first-line therapy failed.41,42 It interacts with transmembrane domain of TPO, which stimulates the proliferation and differentiation of megakaryocytes.43,44 Aside from the direct effect on promoting platelet production, eltrombopag might have additional immunomodulatory activity. Bao et al reported that eltrombopag and other TPO-RAs improved the suppressive function of regulatory CD4+CD25hi T cells (Tregs) by enhancing release of TGF-β1 as a result of greater platelet turnover.22 Moreover, our results showed that eltrombopag therapy altered the FcγR balance in favor of the inhibitory FcγRIIb on monocytes and corrected the enhanced phagocytic capacity of monocytes in ITP. The decrease in platelet phagocytosis by macrophages after eltrombopag treatment might be a concomitant phenomenon after the recovery of immune tolerance. These underlying mechanisms might account for the eltrombopag-induced prolonged response (platelet counts ≥50 × 109/L sustained for >12 weeks after eltrombopag withdrawal without rescue therapy) observed in the long-term EXTEND clinical trial.45
Regulation of FcγR expression in ITP is sophisticated and differs depending on the cell type. Our previous study showed that HD-DXM could restore the balance of monocyte FcγRs by downregulating FcγRI and IIa expression and upregulating FcγRIIb expression in ITP.18 IVIg also induced a significant increase in FcγRIIb expression on macrophages.17 Another study by Bazin showed that small-size tetramolecular immune complexes containing human Fc fragments and mouse anti-human IgG could significantly inhibit the clearance of antibody-coated platelets by interacting with low-affinity FcγRs in a murine model of ITP.46 Moreover, Asahi reported that H pylori eradication could induce the recovery of platelet counts in a certain part of H pylori–positive patients with ITP, which was associated with a change in FcγR balance toward the inhibitory FcγRIIb on monocytes.19 Nevertheless, the requirement of FcγRIIb expression on splenic macrophages has been challenged as evidence accumulated. Leontypev et al found BALB/c or C57BL/6 FcγRIIb−/− mice all recovered from ITP after IVIg treatment, despite higher doses of IVIg being required for B6 mice.47 More recently, Crow and colleagues observed that IVIg could ameliorate murine-passive ITP in FcγRIIb−/− C57BL/6 mice, 129S mice, and B6129SF1 mice, further indicating FcγRIIb was dispensable for IVIg’s therapeutic activity.48 Despite the aforementioned challenges, it is not appropriate to completely rule out the pathogenic roles of aberrant FcγR profiles in ITP. Murine models used in investigating the relation between FcγRIIb and IVIg were all generated by passive transfusion of platelet antibodies, and they were still imprecise models of the human ITP for a variety of reasons. Thus, FcγRIIb upregulation in ITP might be a common phenomenon in the recovering process induced by therapeutic agents, and the precise mechanism of FcγR modulation in ITP still needs further exploration.
In conclusion, eltrombopag was an effective and well-tolerated regimen for the management of corticosteroid-resistant or -relapsed patients with ITP. The recovery of platelet counts after TPO-RA treatment in responders was accompanied by a reversal of the FcγR balance toward the inhibitory FcγRIIb, which might enhance the threshold for monocyte activation and reduce the phagocytic capacity of monocytes. Although these results still need further validation in a longitudinal study, our present data indicate that TPO-RAs have profound effects on immune modulation in ITP.
Part of this study was orally presented at the 19th Congress of the European Hematology Association, Milan, Italy, June 12-15, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alexandra Marshall from the St. Michael’s Hospital, University of Toronto, for language editing of the manuscript.
This work was supported by grants from the Major Research plan of the National Natural Science Foundation of China (No. 91442204), National Natural Science Foundation for Distinguished Young Scholars of China (No. 81125002), National Natural Science Foundation of China (No. 81200344, 81570103, 81270578, 81370623, 81470284), State Program of National Natural Science Foundation of China for Innovative Research Group (81321061), State Key Clinical Specialty of China for Blood Disorders, Tai Shan Scholar Foundation, and Tianjin Municipal Science and Technology Commission (15JCZDJC35800) (L.Z.).
Authorship
Contribution: X.-g.L. and J.P. designed research, performed research, analyzed data, and wrote the paper; S.L., Q.F., X.-n.L., G.-s.L., Z.S., P.C., Y.L., Y.W., X.-y.D., and P.Q. performed research and analyzed data; C.G. and C.M. performed research; and L.Z. and M.H. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, Jinan, 250012, China; email: junpeng88@sina.com.cn; Lei Zhang, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Disease Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 288 Nanjing Road, Tianjin 300020, China; e-mail: zlpumc@hotmail.com; and Ming Hou, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Jinan 250012, China; e-mail: qlhouming@sina.com.cn.
References
Author notes
X.-g.L., S.L., and Q.F. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal