Key Points
Antibody-induced shedding of platelet GPVI in vivo and the associated transient thrombocytopenia depend on liver sinusoidal endothelial cell-expressed FcγRIIB.
Abstract
The activating platelet collagen receptor glycoprotein VI (GPVI) is a promising antithrombotic target because of its central role in arterial thrombosis and its minor relevance for normal hemostasis. The receptor can be specifically targeted by antibodies and irreversibly downregulated in circulating platelets in vivo, resulting in long-term antithrombotic protection in mice. This GPVI immunodepletion predominantly occurs through ectodomain shedding, which is accompanied by a transient drop in peripheral platelet counts. Mechanistic studies on this targeted GPVI loss have been hampered because it cannot be reproduced in isolated platelets in vitro. Here we show that both the transient thrombocytopenia and GPVI ectodomain shedding depend on the Fc portion of the anti-GPVI antibody and its interaction with the inhibitory Fcγ receptor (FcγR)IIB. In wild-type, but not Fcgr2b−/− mice, anti–GPVI-opsonized platelets became transiently trapped in the liver followed by the appearance of the soluble GPVI ectodomain in the plasma. Depletion of Kupffer cells neither affected anti–GPVI-induced platelet accumulation nor GPVI shedding, demonstrating that the other major FcγRIIB-expressing cell type, liver sinusoidal endothelial cells, is required for both processes to occur. These results reveal a novel and unexpected function of hepatic FcγRIIB in the targeted downregulation of GPVI in vivo.
Introduction
The platelet collagen receptor glycoprotein VI (GPVI) is a promising pharmacological target because its absence or functional inhibition provides protection from thrombosis and stroke in several mammalian model organisms1-3 without causing major bleeding.4 Likewise, GPVI deficiency in humans resulting from genetic defects5,6 or autoimmunity does not cause a bleeding diathesis.7,8 GPVI can be efficiently depleted in circulating platelets in mice and humans by antibodies.9 The major route of GPVI immunodepletion is ectodomain shedding, which requires intact GPVI signaling and is accompanied by a transient drop in platelet count.10 Under conditions of defective GPVI signaling, the receptor is still downregulated in vivo, but this occurs considerably more slowly through internalization/intracellular degradation, in the absence of thrombocytopenia. Importantly, however, although different anti-GPVI antibodies efficiently deplete the receptor in vivo in mice, they do not cause GPVI downregulation in vitro.11
The Fc portion of immunoglobulin G (IgG) interacts with Fcγ receptors (FcγRs) on different cells and thereby triggers immune effector functions in vivo. In mice, 3 activating (FcRγ-chain/immunoreceptor tyrosine-based activation motif–linked) FcγRs exist, namely FcγRI, FcγRIII, and FcγRIV, and 1 immunoreceptor tyrosine-based inhibitory motif –bearing inhibitory receptor, FcγRIIB.12 FcγRIIB, which binds IgG immune complexes, but not monomeric IgG, is coexpressed with the activating FcγRs in different immune cells, such that the balance in their expression determines the strength of FcγR activation in response to IgG-immune complexes. However, the majority of FcγRIIB is found in liver sinusoidal endothelial cells (LSECs), which lack activating FcγRs.13 LSEC-FcγRIIB is the critical scavenging receptor mediating capture and removal of viruses and soluble immune complexes.13,14 However, a role of FcγRIIB in the processing of antibody-opsonized blood cells including platelets has not been reported.
Study design
Mice
Knockout mice for GPVI (Gp6−/−) FcγRIIB (Fcgr2b−/−) and FcγRIII (Fcgr3−/−) were described previously.15-17 Animal studies were approved by the district government of Lower Franconia.
Reagents
Streptavidin-horseradish peroxidase (DAKO), clodronate (clodronateliposomes.com), and rabbit anti-rat-IgG-fluorescein isothiocyanate (Dianova) were purchased. JAQ1 (anti-GPVI) and 2.4G2 (anti-CD16/32) were produced and modified in our laboratory.
Ex vivo soluble GPVI enzyme-linked immunosorbent assay and flow cytometry
Experiments were performed as described previously.10
Whole-body in vivo imaging of mice
Circulating platelets were labeled with 0.3 µg/g body weight (BW) anti-GPIXAF750 antibody IV. Four hours later, mice were anesthetized with medetomidine (Pfizer; 0.5 µg/g), midazolam (Roche Pharma AG; 5 µg/g BW), and fentanyl (Janssen-Cilag GmbH; 0.05 µg/g BW). Subsequently, JAQ1 (2 µg/g BW) or vehicle was injected IV and in vivo imaging was performed on an IVIS spectrum device (Perking Elmer) at 800 nm (emission filter).
Immunohistochemistry and IF staining of liver cryo sections
Liver samples were embedded in Tissue-Tek (Sakura Finetek) and snap-frozen in liquid nitrogen. Unspecific binding was blocked with 3% BSA/PBS.
Immunohistochemistry stainings were performed as described previously.18
Immunofluorescence (IF) stainings were performed as follows. Samples were stained with the indicated fluorophore-conjugated antibodies, counterstained with 4′,6-diamidino-2-phenylindole and visualized using confocal microscopy (Leica TCS-SP5). Images were processed in a blinded manner by thresholding, generation of binaries, using the “particle analyzer” (ImageJ 1.50b, National Institutes of Health) platelets, and JAQ1Dy488 “clusters” were quantified.
Statistics
Results from at least 3 experiments per group are presented as mean ± standard deviation. Differences between 2 groups were assessed by Welch t-test. *P < .05; **P < .01; ***P < .001.
Results and discussion
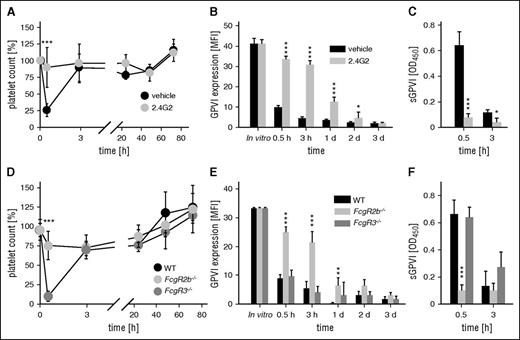
As reported previously,10,11 the anti-GPVI antibody, JAQ1, induced a rapid loss of platelet surface GPVI and the appearance of soluble GPVI (sGPVI) in the plasma, which was accompanied by a transient thrombocytopenia (see supplemental Figure 1, available on the Blood Web site). In sharp contrast, JAQ1-F(ab′)2 fragments neither caused thrombocytopenia (supplemental Figure 1A) nor elevated plasma sGPVI levels (supplemental Figure 1C), demonstrating that both processes are Fc-dependent. A potential involvement of FcγRs was assessed by treating mice with the antibody 2.4G2, which blocks FcγRIII and IIB,19 24 hours before JAQ1 injection. The 2.4G2 abolished both anti–GPVI-induced thrombocytopenia and generation of sGPVI (Figure 1). To determine which of the 2 FcγRs was required for the anti-GPVI effects, we analyzed Fcgr3−/− and Fcgr2b−/− mice. Surprisingly, although a lack of FcγRIII had no effect on JAQ1-induced thrombocytopenia or generation of sGPVI (Figure 1D-F), both processes were abolished in FcgR2b−/− mice, resulting in delayed GPVI downregulation (Figure 1E), presumably reflecting internalization/degradation.10
FcγRIIB, but not FcγRIII, mediates anti–GPVI-induced thrombocytopenia and the generation of sGPVI. (A) Platelet counts of 2.4G2 (gray) or vehicle-treated (black) mice were monitored upon injection of the anti-GPVI antibody JAQ1biotin by flow cytometry. (B) Expression of GPVI on the platelet surface was determined by flow cytometry using an anti-rat IgG-fluorescein isothiocyanate (FITC) antibody. (C) Plasma levels of sGPVI) were determined using an enzyme-linked immunosorbent assay (ELISA) system. (D) Platelet counts of mice lacking FcγRIII (Fcgr3−/−), FcγRIIB (Fcgr2b−/−), or control mice (WT) were monitored upon injection of the anti-GPVI antibody JAQ1biotin by flow cytometry. (E) Expression of GPVI on the platelet surface was determined by flow cytometry using an anti-rat IgG-FITC antibody. (F) Plasma levels of soluble GPVI (sGPVI) were determined using an ELISA system. Data are expressed as mean ± standard deviation (n = 4) and are representative of 3 individual experiments.
FcγRIIB, but not FcγRIII, mediates anti–GPVI-induced thrombocytopenia and the generation of sGPVI. (A) Platelet counts of 2.4G2 (gray) or vehicle-treated (black) mice were monitored upon injection of the anti-GPVI antibody JAQ1biotin by flow cytometry. (B) Expression of GPVI on the platelet surface was determined by flow cytometry using an anti-rat IgG-fluorescein isothiocyanate (FITC) antibody. (C) Plasma levels of sGPVI) were determined using an enzyme-linked immunosorbent assay (ELISA) system. (D) Platelet counts of mice lacking FcγRIII (Fcgr3−/−), FcγRIIB (Fcgr2b−/−), or control mice (WT) were monitored upon injection of the anti-GPVI antibody JAQ1biotin by flow cytometry. (E) Expression of GPVI on the platelet surface was determined by flow cytometry using an anti-rat IgG-FITC antibody. (F) Plasma levels of soluble GPVI (sGPVI) were determined using an ELISA system. Data are expressed as mean ± standard deviation (n = 4) and are representative of 3 individual experiments.
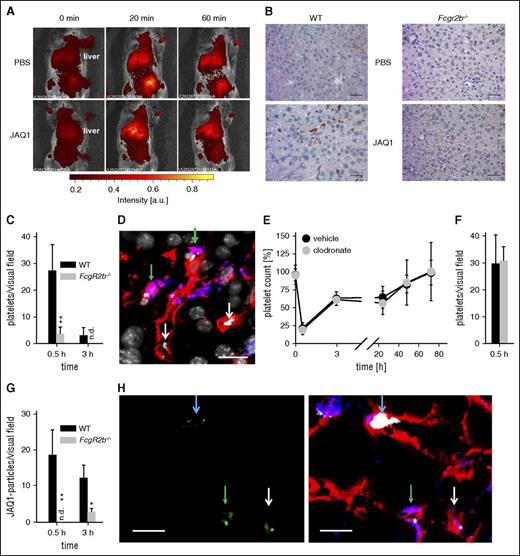
Anti-GPVI opsonized platelets accumulated in the liver of wild-type (WT), but not of FcgR2b−/−, mice (Figure 2A-D). Remarkably, only one-third of the “trapped” WT platelets was in close proximity to Kupffer cells (Figure 2D, green arrows), whereas virtually all platelets were found close to the endothelium. Kupffer cell depletion with clodronate liposomes20 had no effect on anti–GPVI-induced thrombocytopenia, platelet accumulation in the liver, or sGPVI plasma levels (Figure 2E-F; supplemental Figure 2). Furthermore, we detected robust signals for clusters of JAQ1Dy488 in liver sections of WT, but not FcgR2b−/− mice (Figure 2G-H and data not shown), which likely reflected large sGPVI-JAQ1 complexes. Notably, some of the JAQ1Dy488 signal colocalized with platelets (Figure 2H, blue arrows), whereas clusters of nonplatelet-associated antibodies were also found within endothelial or Kupffer cells (Figure 2H), likely representing sGPVI-JAQ1 complexes released from the platelet surface.
JAQ1-opsonized platelets are “trapped” primarily in the liver of WT, but not FcγRIIB-deficient mice. (A) Sequestration of fluorescently labeled platelets was monitored in anesthetized mice upon injection of JAQ1 or vehicle using an in vivo imaging system. (B) Sections of snap-frozen liver samples of WT mice and mice lacking FcγRIIB (Fcgr2b−/−) were probed with horseradish peroxidase–conjugated anti-GPIb antibodies. Detection was performed using 3-Amino-9-ethylcarbazole. (C-H) For confocal microscopy, liver sections of WT mice treated with DyLight488-conjugated JAQ1 (green) 30 min before organ extraction were stained for platelets (anti-GPIX, light blue), Kupffer cells (F4/80, blue), and endothelium (anti-CD105, red), and counterstained by 4′,6-diamidino-2-phenylindole (gray). (C) Platelets per visual field (388 × 388 µm) were quantified in liver sections of WT and FcgR2b−/− mice (4-5 sections per animal, n ≥ 3; n.d., not detectable). (D) Platelets (light blue) can be detected in close proximity to the endothelium (white arrows) or Kupffer cells (green arrows). Scale bar, 20 µm. (E) Platelet counts of mice treated with clodronate liposomes (clodronate) or PBS liposomes (vehicle) 48 h before the experiment were monitored upon injection of the anti-GPVI antibody JAQ1biotin by flow cytometry. (F) Platelets per visual field were quantified in liver sections of clodronate and vehicle liposome-treated mice (4-5 sections per animal, n = 3). (G) JAQ1Dy488-clusters per visual field (388 × 388 µm) were quantified in liver sections of WT and FcgR2b−/− mice (4-5 sections per animal, n ≥ 3; n.d., not detectable). (H) JAQ1Dy488 (green, left panel = isolated channel, right panel = merge) can be detected in WT liver sections attached to some platelets (light blue arrow), engulfed by Kupffer cells (green arrows) or endothelial cells (white arrows). Scale bar, 10 µm.
JAQ1-opsonized platelets are “trapped” primarily in the liver of WT, but not FcγRIIB-deficient mice. (A) Sequestration of fluorescently labeled platelets was monitored in anesthetized mice upon injection of JAQ1 or vehicle using an in vivo imaging system. (B) Sections of snap-frozen liver samples of WT mice and mice lacking FcγRIIB (Fcgr2b−/−) were probed with horseradish peroxidase–conjugated anti-GPIb antibodies. Detection was performed using 3-Amino-9-ethylcarbazole. (C-H) For confocal microscopy, liver sections of WT mice treated with DyLight488-conjugated JAQ1 (green) 30 min before organ extraction were stained for platelets (anti-GPIX, light blue), Kupffer cells (F4/80, blue), and endothelium (anti-CD105, red), and counterstained by 4′,6-diamidino-2-phenylindole (gray). (C) Platelets per visual field (388 × 388 µm) were quantified in liver sections of WT and FcgR2b−/− mice (4-5 sections per animal, n ≥ 3; n.d., not detectable). (D) Platelets (light blue) can be detected in close proximity to the endothelium (white arrows) or Kupffer cells (green arrows). Scale bar, 20 µm. (E) Platelet counts of mice treated with clodronate liposomes (clodronate) or PBS liposomes (vehicle) 48 h before the experiment were monitored upon injection of the anti-GPVI antibody JAQ1biotin by flow cytometry. (F) Platelets per visual field were quantified in liver sections of clodronate and vehicle liposome-treated mice (4-5 sections per animal, n = 3). (G) JAQ1Dy488-clusters per visual field (388 × 388 µm) were quantified in liver sections of WT and FcgR2b−/− mice (4-5 sections per animal, n ≥ 3; n.d., not detectable). (H) JAQ1Dy488 (green, left panel = isolated channel, right panel = merge) can be detected in WT liver sections attached to some platelets (light blue arrow), engulfed by Kupffer cells (green arrows) or endothelial cells (white arrows). Scale bar, 10 µm.
Analysis of bone marrow chimeric mice lacking FcγRIIB either in the hematopoietic or in the nonhematopoietic compartment (ie, the endothelium) demonstrated that the absence of endothelial FcγRIIB ameliorated the anti–GPVI-induced thrombocytopenia and resulted in a delayed loss of surface GPVI and reduced generation of sGPVI (supplemental Figure 3B-C) independently of the expression of the receptor in hematopoietic cells (supplemental Figure 3A).
Our results reveal a novel role of the inhibitory FcγRIIB in processing anti–GPVI-opsonized platelets. Based on the results obtained in Kupffer cell depleted and in bone marrow chimeric mice, we conclude that LSECs are the critical cell type to execute this function. The (transient) thrombocytopenia promoting effect of FcγRIIB was unexpected because this receptor is best known for its inhibitory action on either activating FcγRs or B-cell receptors.21 Given the differences in FcγR expression between mice and humans,12 further studies will be required to assess the exact nature and potential IgG subclass specificity of FcγRs mediating GPVI-immunodepletion in humans.
It is unclear at present how the interaction between FcγRIIB and antibody-opsonized platelets would result in the quantitative loss of platelet GPVI. However, the high abundance of FcγRIIB on LSECs and its capacity to cluster anti-GPVI antibodies likely provides a sufficient cross-linking array necessary for platelet binding and activation that in turn results in the generation of sGPVI. Combined loss of ADAM17 and ADAM10 in platelets prevents GPVI shedding in vitro, but not in vivo.11 Shedding in trans may occur, because it has been described for ADAM10-mediated processing of ephrin-5a.22 However, the production of sGPVI in mice constitutively lacking ADAM1711 or ADAM10 in endothelial cells (supplemental Figure 4) was unaltered. Thus, the identity of the protease(s) responsible for the generation of sGPVI in vivo remains to be determined.
Taken together, our data reveal for the first time an unexpected function of LSEC-FcγRIIB acting in trans for the targeted GPVI ectodomain shedding in vivo, at least in mice. These results may have an impact on the development of antibody-based anti-platelet therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Juliana Goldmann and Stefanie Hartmann for excellent technical assistance, Deya Cherpokova for critical comments, and the Bio-Imaging Center for providing technical infrastructure.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 688) and the Rudolf Virchow Center.
Authorship
Contribution: D.S., M.P., V.L., and J.K.W. performed experiments and analyzed data; D.S. and J.E.G. interpreted results and contributed to the writing of the manuscript; J.E.G. provided vital reagents; and B.N. designed research, funded the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing interests.
Correspondence: Bernhard Nieswandt, Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal