The in vivo mechanisms underlying antibody-based shedding of the key platelet collagen receptor glycoprotein VI (GPVI) are potentially significant for future antibody-based thromboprotective agents. In this issue of Blood, Stegner et al reveal how the inhibitory Fc receptor, FcγRIIB, on mouse liver sinusoidal endothelium mediates both antibody-induced GPVI shedding and entrapment of platelets in the liver.1
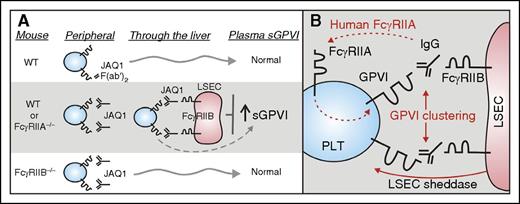
Role of FcγRIIB in regulating antibody-induced ectodomain shedding of platelet GPVI. (A) New studies (Stegner et al) have now shown that depletion of platelet surface GPVI by the anti-GPVI antibody JAQ1 depends on expression of an inhibitory Fc receptor, FcγRIIB, expressed on LSEC. WT mice or mice deficient in the activating Fc receptor, FcγRIIA (FcγRIIA−/−), treated with JAQ1 showed entrapment of platelets in the liver and increased GPVI shedding as demonstrated by elevated levels of sGPVI in plasma. In contrast, WT mice treated with JAQ1 F(ab′)2 fragments or mice deficient in FcγRIIB (FcγRIIB−/−) did not show these effects. (B) Human, but not mouse, platelets express FcγRIIA providing a potential pathway for antibody-induced GPVI shedding in peripheral blood (dashed line), whereas potential mechanisms for antibody-induced GPVI shedding in mice mediated by FcγRIIB in the liver might involve FcγRIIB/IgG-dependent clustering of GPVI and involvement of a sheddase derived from LSEC. LSEC, liver sinusoidal endothelial cells; PLT, platelet; WT, wild-type.
Role of FcγRIIB in regulating antibody-induced ectodomain shedding of platelet GPVI. (A) New studies (Stegner et al) have now shown that depletion of platelet surface GPVI by the anti-GPVI antibody JAQ1 depends on expression of an inhibitory Fc receptor, FcγRIIB, expressed on LSEC. WT mice or mice deficient in the activating Fc receptor, FcγRIIA (FcγRIIA−/−), treated with JAQ1 showed entrapment of platelets in the liver and increased GPVI shedding as demonstrated by elevated levels of sGPVI in plasma. In contrast, WT mice treated with JAQ1 F(ab′)2 fragments or mice deficient in FcγRIIB (FcγRIIB−/−) did not show these effects. (B) Human, but not mouse, platelets express FcγRIIA providing a potential pathway for antibody-induced GPVI shedding in peripheral blood (dashed line), whereas potential mechanisms for antibody-induced GPVI shedding in mice mediated by FcγRIIB in the liver might involve FcγRIIB/IgG-dependent clustering of GPVI and involvement of a sheddase derived from LSEC. LSEC, liver sinusoidal endothelial cells; PLT, platelet; WT, wild-type.
As both an antithrombotic target and platelet-specific biomarker, GPVI of the immunoreceptor family stands out because of: (1) its restricted expression on megakaryocytes/platelets, (2) its key role on platelets as a primary receptor for collagen and fibrin,2,3 and (3) experimental findings that GPVI-deficiency in mouse models markedly limits occlusive thrombosis and stroke, with more minimal hemostatic effects on tail-bleeding.4 Furthermore, the shed soluble ectodomain of GPVI or soluble GPVI (sGPVI) shows increasing promise as a platelet-specific biomarker for platelet-related dysfunction in human disease.5 GPVI (∼62 kDa) consisting of 2 extracellular immunoglobulin-like domains and a short mucin domain, a transmembrane domain, and a cytoplasmic tail, forms a noncovalent complex with the Fc receptor γ-chain (FcRγ) essential for GPVI surface expression and intracellular signaling. FcRγ contains a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM), which, upon ligand-induced crosslinking of GPVI/FcRγ, activates Syk kinase signaling pathways.
An important regulatory mechanism controlling GPVI surface expression is metalloproteinase-mediated ectodomain shedding, releasing an ∼55-kDa soluble shed fragment (ie, sGPVI). GPVI shedding involves one or more platelet-expressed metalloproteinases, predominantly ADAM10. Basic mechanisms of GPVI shedding from platelets include triggers such as ligand binding, elevated shear forces and coagulation, as well as anti-GPVI or antiplatelet antibodies.6 In human platelets, anti-GPVI murine monoclonal antibodies (mAbs), human autoantibodies from individuals with immune thrombocytopenia, or other immune complexes can induce loss of surface GPVI and release sGPVI from healthy donor platelets in vitro.7 One of the underlying mechanisms may involve the activation of the low-affinity immunoglobulin G (IgG) receptor, FcγRIIA, which activates platelets via an intracellular ITAM.7 sGPVI is also significantly elevated in the plasma of some, though not all, cases of immune thrombocytopenia.8 Interestingly, mouse platelets that lack expression of FcγRIIA exhibit antibody-induced decrease of platelet surface GPVI and elevated plasma sGPVI when treated by in vivo perfusion, but not in vitro, with JAQ1, a mAb against GPVI.9 Treatment with JAQ1 leads to depletion of platelet GPVI associated with long-term antithrombotic protection and with minimal effects on hemostasis. JAQ1-induced loss of GPVI predominantly involves ectodomain shedding, and is Fc dependent, because the F(ab′)2 fragment of JAQ1 lacking Fc does not deplete GPVI, and loss of GPVI is prevented by inhibitors of the Fc receptors, FcγRIIA and FcγRIIB.
The new studies (see figure panel A for summary) demonstrate that JAQ1-induced shedding of platelet GPVI depends on expression of an inhibitory Fc receptor, FcγRIIB, which contains an immunoreceptor tyrosine-based inhibitory motif, and is expressed on LSEC. WT mice or mice deficient in FcγRIIA (FcγRIIA−/−) treated with JAQ1 showed entrapment of platelets in the liver resulting in transient thrombocytopenia and increased GPVI shedding as demonstrated by elevated levels of sGPVI in plasma. In contrast, mice deficient in FcγRIIB (FcγRIIB−/−) did not show these effects (see figure panel A). How this shedding occurs requires further investigation. However, potential mechanisms for antibody-induced GPVI shedding in mice mediated by FcγRIIB in the liver might involve FcγRIIB/IgG-dependent clustering of GPVI and/or the possible involvement of a sheddase derived from LSEC (see figure panel B). In future studies, it would be worthwhile to examine heterozygous forms of FcγRIIB deficiency where the receptor is expressed but in reduced amounts to assess any expression density dependency relevant to a mechanism involving crosslinking. It would also be interesting to assess whether antibody-based depletion of other platelet receptors such as CLEC-2 (INU1) shows similar dependence on LSEC FcγRIIB expression. Because human but not mouse platelets express FcγRIIA, which may provide an alternative pathway for antibody-induced GPVI shedding in human peripheral blood (see figure panel B), it remains to be established whether activation-dependent or activation-independent immune clearance of human platelets in nonobese diabetic/SCID mice10 involves LSEC and an FcγRIIB-dependent mechanism, and indeed whether this mechanism is relevant to immune thrombocytopenia in general.
This present study is potentially significant for understanding mechanisms underlying antibody-based methods of platelet GPVI depletion for future thromboprotective applications. As mentioned earlier, some but not all cases of human immune thrombocytopenia have elevated plasma sGPVI, and this liver-based mechanism could also have important implications for other antibody-based therapies. Finally, these studies raise questions surrounding differences in mammalian evolution and immunologic development. That is, why mouse platelets have no FcγRIIA and response to antiplatelet antibodies occurs at the liver endothelium, whereas humans have additional peripheral responsiveness capacity toward antiplatelet antibodies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.