Abstract
In recent years, the traditional view of the hemostatic system as being regulated by a coagulation factor cascade coupled with platelet activation has been increasingly challenged by new evidence that activation of the immune system strongly influences blood coagulation and pathological thrombus formation. Leukocytes can be induced to express tissue factor and release proinflammatory and procoagulant molecules such as granular enzymes, cytokines, and damage-associated molecular patterns. These mediators can influence all aspects of thrombus formation, including platelet activation and adhesion, and activation of the intrinsic and extrinsic coagulation pathways. Leukocyte-released procoagulant mediators increase systemic thrombogenicity, and leukocytes are actively recruited to the site of thrombus formation through interactions with platelets and endothelial cell adhesion molecules. Additionally, phagocytic leukocytes are involved in fibrinolysis and thrombus resolution, and can regulate clearance of platelets and coagulation factors. Dysregulated activation of leukocyte innate immune functions thus plays a role in pathological thrombus formation. Modulation of the interactions between leukocytes or leukocyte-derived procoagulant materials and the traditional hemostatic system is an attractive target for the development of novel antithrombotic strategies.
Introduction
Hemostatic thrombus formation is conventionally thought to involve a coagulation factor cascade coupled with platelet activation. Pathological thrombosis, as described in the 1850s by the German pathologist Rudolph Virchow, is influenced by aberrant activation of coagulation, disruption of the vessel wall, and stasis.1 However, in recent years this model has undergone a significant paradigm shift due to accumulating evidence of an intrinsic link between the coagulation and innate inflammatory systems. The term immunothrombosis, coined in 2013 by Engelmann and Massberg,2 formalized this concept and described a process by which the activation of coagulation assists the function of the innate immune system, and the converse, whereby components of the immune system contribute to thrombosis. Dysregulated activation of the immune system can thus contribute to the genesis of pathological macro- and microvascular thrombosis.
The contribution of leukocytes to coagulation is a subject of both longstanding interest as well as current intensive study. With the development of intravital imaging techniques, animal models that closely mimic the pathogenesis of thrombosis in humans, and selective antagonists of leukocyte-regulated procoagulant pathways, the role that leukocytes play in regulating thrombosis is being unveiled. Leukocytes, namely monocytes, macrophages, and neutrophils, express and release coagulation and fibrinolytic factors, and interact with the hemostatic system through innate immune functions. Leukocytes produce cytokines that modulate the expression of procoagulant and adhesive molecules on vascular endothelial cells. Antimicrobial agents released during leukocyte degranulation and extracellular trap formation directly activate platelets and the coagulation cascade. Additionally, leukocyte chemotaxis and phagocytic functions regulate thrombus resolution. In this review, the influence of leukocytes on blood coagulation and platelet activation will be described, and evidence assessing the contribution of leukocytes to venous, arterial, and microvascular thrombosis will be considered.
Leukocytes regulate the coagulation cascade
Under normal physiological circumstances, quiescent leukocytes promote the maintenance of blood fluidity. For example, circulating monocytes express the anticoagulant factors endothelial protein C receptor (EPCR),3 thrombomodulin (TM),4 and tissue factor pathway inhibitor (TFPI).5 However, under proinflammatory or apoptotic conditions, leukocytes can rapidly undergo a phenotypic transformation, synthesizing and secreting procoagulant factors or agents that activate coagulation (Figure 1). Additionally, the leukocyte cell surface can provide a site for coagulation factor assembly and activation.6,7
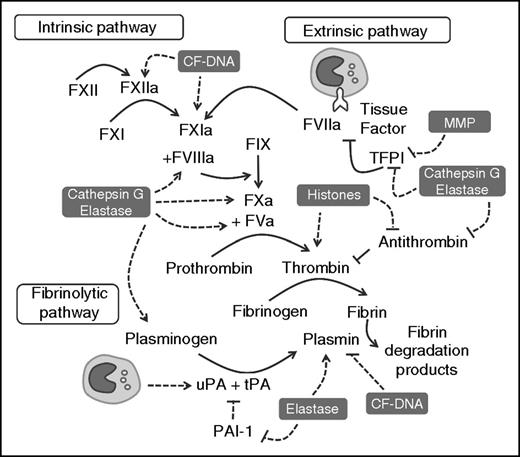
Leukocyte-released enzymes and DAMPs interact with components of the coagulation cascade. CF-DNA, histones, MMP, cathepsin G, and/or elastase modulate the traditional coagulation cascade by facilitating the activation of zymogen coagulation and fibrinolytic factors, and inhibiting the activity of endogenous anticoagulants. TF expressed by circulating leukocytes can activate coagulation through the extrinsic pathway, whereas monocyte-derived uPA can modulate fibrinolysis. CF-DNA, cell-free DNA; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; uPA, urokinase-type plasminogen activator.
Leukocyte-released enzymes and DAMPs interact with components of the coagulation cascade. CF-DNA, histones, MMP, cathepsin G, and/or elastase modulate the traditional coagulation cascade by facilitating the activation of zymogen coagulation and fibrinolytic factors, and inhibiting the activity of endogenous anticoagulants. TF expressed by circulating leukocytes can activate coagulation through the extrinsic pathway, whereas monocyte-derived uPA can modulate fibrinolysis. CF-DNA, cell-free DNA; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; uPA, urokinase-type plasminogen activator.
Tissue factor (TF)
Monocytes are the largest intravascular source of TF.8,9 Although low levels of TF antigen are detected on quiescent monocytes, exposure to agents that promote inflammation, and/or apoptosis, including high mobility group box-1 (HMGB-1),10 chemotherapy,11 lipopolysaccharide,12 hypoxia,13 and anti-HIT antibodies14 increase monocyte TF activity and/or TF-mediated thrombin generation. Monocyte TF activity is regulated by processes that increase TF expression, induce TF decryption,15 and modulate the balance between TF and TFPI.16 Activated monocytes also shed microparticles from P-selectin glycoprotein ligand 1 (PSGL-1)–rich membrane microdomains17 that carry TF, phosphatidylserine, and other regulators of coagulation.18 Expression of TF by other leukocyte subtypes is more controversial. Although quiescent neutrophils likely do not express TF antigen,19 TF can be expressed in smaller quantities by neutrophils stimulated ex vivo20,21 and in animal models.22,23 This may in part be the result of neutrophil acquisition of TF from monocytes,24 potentially through a process involving microparticle-mediated transfer. Similarly, conflicting reports describe the expression of TF by eosinophils, but may partially explain the increased risk for thrombosis in eosinophilia.25-27
Granular enzymes
Neutrophils, and to a lesser extent monocytes, and basophils release matrix metalloproteinases and serine proteases such as cathepsin G and elastase, from cytoplasmic granules in response to stimulation.28,29 These enzymes promote coagulation activation through numerous mechanisms (Figure 1), including directly activating cofactors factor V (FV),30 FVIII,31 and zymogen FX.32 They can also degrade anticoagulant factors such as antithrombin,33 heparin cofactor II,34 and/or TFPI.35-37
Nuclear damage-associated molecular patterns (DAMPs)
DAMPs, including DNA, HMGB1, and histones are released from the nuclei of activated or apoptotic leukocytes and promote the activation of coagulation. The release of neutrophil chromatin as neutrophil extracellular traps (NETs) can be triggered by exposure to microorganisms, activated platelets, inflammatory cytokines, and HMGB1.38-41 As well, NETs have also been reported to be released by monocytes/macrophages42 and mast cells,43 whereas basophils44 and eosinophils45 release extracellular traps comprised of granular enzymes and mitochondrial DNA. CF-DNA may also be released from apoptotic or necrotic cells in the circulation or vessel wall.46
Intact NETs act as a scaffold that concentrates procoagulant effectors including platelets, red blood cells, von Willebrand factor (VWF), TF, protein disulfide isomerase, HMGB1, cathepsin G, elastase, fibrin(ogen), and fibronectin.22,47,48 The influence of NET components on coagulation activation has been independently evaluated (Figure 1). Extracellular DNA triggers contact pathway activation through FXI and FXII.49,50 Histone H4 binds to prothrombin and generates thrombin by auto-activation.51 DAMPs can also inhibit anticoagulant pathways by protecting thrombin from antithrombin-mediated inactivation,52,53 and by impairing protein C activation by thrombin-TM.10,54
Leukocytes modulate the hemostatic activity of endothelial cells and platelets
Leukocyte-released antimicrobial enzymes, cytokines, and DAMPs, can modulate the anticoagulant activity of endothelial cells (Figure 2). Cytokines such as tumor necrosis factor-α and interleukin-1β can downregulate expression of EPCR and TM through decreased messenger RNA synthesis,55 and increased EPCR shedding.56 Histones are cytotoxic to endothelial cells,57 and increase surface phosphatidyserine exposure on eryothrocytes.58 Histones,59 cytokines,60 HMGB1,10 and granular enzymes61 can increase in vitro endothelial cell TF activity. Histones and cytokines can also stimulate the exocytosis of endothelial Weibel-Palade bodies, inducing the release of VWF and/or P-selectin.62,63 Activated basophils also release histamine,29 a potent secretagogue for VWF.64 Additionally, both cytokines and neutrophil-generated oxidants such as HOCl impair cleavage of VWF by the protease ADAMTS13, potentially increasing the proportion of circulating ultralarge VWF multimers with enhanced platelet-binding abilities.63,65
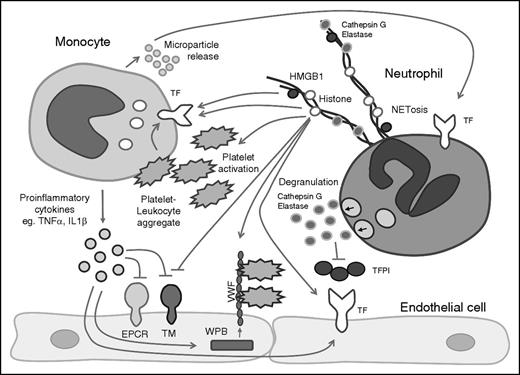
Leukocyte-released enzymes, DAMPs, and cytokines regulate the hemostatic activity of endothelial cells and platelets. Mediators released by activated leukocytes can influence the hemostatic activity of the endothelium and platelet activation. WPB, Weibel-Palade body.
Leukocyte-released enzymes, DAMPs, and cytokines regulate the hemostatic activity of endothelial cells and platelets. Mediators released by activated leukocytes can influence the hemostatic activity of the endothelium and platelet activation. WPB, Weibel-Palade body.
Although activated platelets can stimulate NETosis,41 leukocytes interact with activated platelets to form heterotypic leukocyte-platelet aggregates (Figure 3B). Exposure to proinflammatory and procoagulant stimuli, as well as high shear stress have been shown to promote the formation of these complexes.66-69 Heterotypic aggregates form when activated, and degranulated platelets expose P-selectin on their surface that binds to leukocyte surface PSGL-1.70 Signaling through PSGL-1 rapidly upregulates leukocyte expression of αMβ271 that binds platelet GPIbα72 or GPIIbIIIa via a fibrinogen intermediate (Figure 3B).73 The complexes are further stabilized by multiple receptor-ligand interactions including CD40-CD40 ligand,74 extracellular matrix metalloproteinase inducer-glycoprotein VI (GPVI),75 lymphocyte function-associated antigen 1-ICAM-2,76 and junctional adhesion molecule-C-αMβ2.77 PSGL-1 engagement can also activate cooperative signaling through NF-κB to induce the production of proinflammatory cytokines.78 Although conflicting reports exist, evidence suggests that platelet P-selectin interactions with leukocyte PSGL-1 may also activate TF on monocytes and/or neutrophils.20,78,79 Increased levels of leukocyte-platelet aggregates are frequently associated with thromboinflammatory disorders, and can be used as stable markers of underlying hypercoagulability.
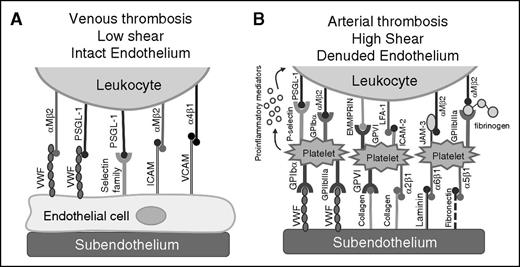
Recruitment of leukocytes to the growing thrombus. Leukocyte recruitment to the thrombus is influenced by the condition of the endothelium and the shear rate in the vessel. (A) Under low shear with intact endothelium, leukocytes are able to bind adhesive molecules on the endothelial surface such as VWF, selectin family members, and cell adhesion molecules to leukocyte-expressed PSGL-1 or integrin receptors. (B) In arterial thrombosis, leukocyte recruitment to the thrombus is influenced by platelet binding, where glycoprotein receptors/β1 and β3 integrins on activated platelets bind to subendothelial adhesion molecules such as VWF, collagen, laminin, and fibronectin. Leukocytes bind to adhered platelets in a PSGL-1/P-selectin–dependent manner and are further stabilized by additional ligand-receptor interactions. EMMPRIN, extracellular matrix metalloproteinase inducer; JAM-3, junctional adhesion molecule; LFA-1, lymphocyte function-associated antigen 1.
Recruitment of leukocytes to the growing thrombus. Leukocyte recruitment to the thrombus is influenced by the condition of the endothelium and the shear rate in the vessel. (A) Under low shear with intact endothelium, leukocytes are able to bind adhesive molecules on the endothelial surface such as VWF, selectin family members, and cell adhesion molecules to leukocyte-expressed PSGL-1 or integrin receptors. (B) In arterial thrombosis, leukocyte recruitment to the thrombus is influenced by platelet binding, where glycoprotein receptors/β1 and β3 integrins on activated platelets bind to subendothelial adhesion molecules such as VWF, collagen, laminin, and fibronectin. Leukocytes bind to adhered platelets in a PSGL-1/P-selectin–dependent manner and are further stabilized by additional ligand-receptor interactions. EMMPRIN, extracellular matrix metalloproteinase inducer; JAM-3, junctional adhesion molecule; LFA-1, lymphocyte function-associated antigen 1.
Activated leukocytes can also induce platelet activation and aggregation by releasing potent platelet activators including elastase,80 cathepsin G,81 and platelet activating factor (Figure 2).82 The influence of NETs on platelet activation has been characterized using several in vitro and in vivo models. NETs can bind to both platelets and VWF under shear,47 and stimulation of endothelial cells by histones increases platelet capture by VWF in a flow chamber system.62 This interaction is likely mediated by both increased Weibel-Palade body exocytosis and platelet activation, as both intact NETs50 and extracellular histones induce platelet activation via toll-like receptor 2 (TLR2) and TLR4.83,84 Platelet activation by histones promote the formation of leukocyte-platelet aggregates and induces the release of platelet VWF, P-selectin, and polyphosphate, which promotes platelet-dependent thrombin generation.69,84 In vivo, infusions of extracellular histones promote the formation of platelet-rich microthrombi with concomitant thrombocytopenia.83
Leukocytes contribute to deep vein thrombosis (DVT)
Clinical studies have described evidence of activated leukocytes associated with venous, arterial, and microvascular thrombosis. Elevated levels of circulating markers of NETs and neutrophil activation,85,86 as well as increased monocyte TF,87,88 have been observed in patients with DVTs compared with control subjects. In addition to releasing disseminated procoagulant factors into the blood, it is increasingly recognized that leukocytes also assemble at the site of vascular injury, and are actively incorporated into forming thrombi. Studies evaluating the composition of venous thrombi from humans demonstrated the presence of TF-expressing leukocytes and NETs.89,90 These processes increase the localized concentration of leukocyte-derived procoagulant activity and potentially forms the nidus upon which the thrombus develops.
Experimental animal models have also shown the association of leukocyte recruitment with the induction of venous thrombosis. In the detailed characterization of a mouse inferior vena cava (IVC) stenosis model, the recruitment of leukocytes to the site of venous thrombosis occurred overlying the intact but activated endothelium within an hour of vessel flow restriction.22 Within 6 hours, leukocytes overlay the endothelial surface, with neutrophils and monocytes comprising 70% and 30% of leukocytes within the thrombus, respectively. Leukocyte recruitment may also be facilitated by the release of cytokines and chemokines by activated platelets,91 which bind directly to the endothelium, or form heterotypic aggregates within the venous thrombus.22,92 Leukocyte rolling is mediated by the upregulation of selectins on the endothelial surface that binds leukocyte PSGL-1, and genetic deletion of P- and E-selectins reduces leukocyte accumulation and venous thrombus size (Figure 3A).22,93 Firm attachment of leukocytes can involve endothelial VWF, VCAM, and ICAM binding leukocyte PSGL-1 and integrins.22,94-96
Assessment of the contribution of leukocytes to the genesis of venous thrombosis is dependent on the animal model used. Both neutrophil and monocyte depletion inhibit thrombosis in murine IVC stenosis22 and ferric chloride models,97 respectively. With respect to leukocyte TF, infusion of activated TF-expressing monocytes resulted in systemic venous and arterial thrombosis in rabbits,98 and IVC ligation models have demonstrated the presence of TF-expressing leukocytes within venous thrombi in rabbits99 and rats.100 In the venous stenosis IVC ligation model, selective depletion of hematopoietic TF significantly attenuates thrombus formation22 ; however, in contrast, DVT formation in response to complete IVC stasis does not involve hematopoietic TF.101
Although the majority of leukocyte-released TF associated with venous thrombi is likely derived from monocytes, neutrophils have also been variably implicated in the formation of DVT. For example, NETs are associated with venous thrombi derived from murine and baboon models.102,103 Infusions of extracellular histones promote DVT development in murine IVC stenosis models, whereas the administration of DNAse1, or FXII deficiency attenuates DVT formation.22,103 In contrast, a model of spontaneous venous thrombosis using small interfering RNA knockdown of protein C and antithrombin, showed that although neutrophils were associated with venous thrombi, neutrophil depletion did not diminish thrombus formation.104 Interestingly, protein arginine deiminase 4 (PAD4) and neutrophil elastase, which regulate chromatin decondensation and NETosis, have contradictory influence on DVT induction. Although PAD4 deficiency was associated with impaired thrombus formation in the IVC stenosis model,105 an electrolytic injury model reported no influence of PAD4 deficiency on DVT.106 Additionally, neutrophil elastase deficiency has been shown to impair NET formation in response to microbial infection,107,108 however, a sterile IVC stenosis model demonstrated elastase-deficient mice generate NETs and have normal venous thrombi.109 Although overall these studies appear to support a role for leukocytes in the induction of thrombosis in response to venous stenosis, they also highlight how the variability of the models employed, including the extent of endothelial injury and underlying activation status of circulating leukocytes, can influence the induction of venous thrombosis.
Leukocytes contribute to arterial thrombosis
Leukocytes can participate in atherothrombosis by generating procoagulant material within the atherosclerotic plaque and contributing to the formation of arterial thrombi overlying the site of vessel rupture. Localized exposure of vessel wall procoagulant material, including macrophage/foam cells that express high levels of TF110 and shed TF-positive microparticles,111 is the precipitating event in atherothrombosis. Arterial thrombosis occurs under conditions of high shear, whereby circulating platelets bind subendothelial ligands, such as collagen and VWF that facilitate the generation of a platelet-rich thrombus. Activated platelets release cytokines that can modulate leukocyte activation,41,112 resulting in the formation of heterotypic leukocyte-platelet aggregates that serve to localize activated leukocytes to the arterial thrombus (Figure 3B). Plasma levels of TF-expressing monocytes and microparticles, leukocyte-platelet aggregates, and NET markers are elevated in conditions that predispose to cardiovascular disease.113,114 Histologic analysis of coronary artery and catheter-associated arterial thrombi demonstrated the presence of monocytes, neutrophils, and eosinophils within the thrombi.115,116
Although murine models do not typically display spontaneous atherothrombosis, intravital models of arterial thrombosis address thrombus formation in macrovascular beds in the context of activated or damaged endothelium. Vessel wall TF is unequivocally involved in thrombus initiation in arterial thrombosis,117 however, the role of circulating leukocyte or leukocyte-derived TF regulating arterial thrombus formation is less clear. The inhibition of leukocyte accumulation using an anti–P-selectin antibody in a baboon model of arterial thrombus significantly attenuated fibrin formation and decreased thrombus stability.118 Another study utilizing reciprocal bone marrow transplants between normal and low TF-expressing mice showed no decrease in Rose Bengal-induced carotid artery thrombus formation in healthy mice in the absence of hematopoietic TF.101 However, infusion of microparticles prepared from human monocytes increased fibrin formation in a carotid ligation injury in a TF-dependent manner.119 Because monocyte and microparticle TF activity is generally increased for individuals predisposed to arterial thrombosis, the direct influence of hematopoietic TF on arterial thrombus formation in this context has yet to be clearly elucidated.
The role of neutrophils in the propagation of arterial thrombosis has been characterized in murine carotid artery ferric chloride and ligation models.35 In these studies, elastase/cathepsin G-deficient mice had reduced arterial thrombus formation related to serine-protease degradation of TFPI. Moreover, an anti-H2A-H2B-DNA neutralizing antibody impaired thrombus formation and decreased thrombus stability in normal but not elastase/cathepsin G-deficient mice. This effect may be related to the reduction in co-assembly of elastase, cathepsin G, and TFPI with extracellular nucleosomes,35 although the influence of elastase deficiency on NET formation was not directly evaluated in this model. In animal models, NETs are associated with the lumen overlying the atherosclerotic plaque,120 and are found elaborated with TF within the arterial thrombus, suggesting that NETs may help localize leukocyte-derived TF within the arterial thrombus.121 Interestingly, the administration of DNAse does not attenuate arterial thrombus formation in healthy mice,122 although both DNAse and/or PAD4 inhibition impair arterial thrombosis in murine models of lupus and atherosclerosis, where animals are predisposed to NET formation.123,124
Leukocytes contribute to microvascular thrombosis and disseminated intravascular coagulation (DIC)
Microvascular thrombosis involves the development of thrombi in the venules, arterioles, and capillaries. Individuals with thrombotic microangiopathies arising from non-infectious etiologies display evidence of elevated NET markers125 and impaired DNAse function126 that may contribute to the acute phase of these disorders. In healthy animals, microvascular thrombosis is evaluated most frequently using chemical or laser-induced endothelial injury models of the cremaster and mesenteric arterioles. In these models, it has been demonstrated that TF delivery to the thrombus is mediated through accumulation of both leukocytes23 as well as leukocyte-derived microparticles.127,128 Adhesion of neutrophils to the activated endothelium occurs immediately, and is mediated through lymphocyte function-associated antigen 1 binding to endothelial ICAM-1, whereas monocyte recruitment occurs 3 to 5 minutes post-injury.23 Depletion of neutrophils or impaired neutrophil-endothelial cell interactions diminished TF accumulation and thrombus formation, suggesting that TF-positive neutrophils form a focus for thrombus development.23 Concurrently, TF-expressing microparticles from both vessel wall and leukocyte origin accumulate at the injury in a PSGL-1/P-selectin–dependent manner.128,129 Monocyte and microparticle-associated TF may facilitate thrombus propagation, because in the absence of hematopoietic TF, fibrin deposition throughout the thrombus is diminished.128
In humans, microvascular thrombosis is most frequently associated with DIC caused by endotoxemia, sepsis, or trauma. Evidence suggests that dysregulated activation of leukocytes is associated with sepsis and/or DIC in humans and animal models. Endotoxin and/or microbes can stimulate the expression of TF on monocytes,130,131 formation of leukocyte-derived microparticles,132 neutrophil degranulation, and NETosis41 ex vivo. Patients with sepsis and/or evidence of DIC have evidence of elevated monocyte TF,133 TF-expressing microparticles,134 HMGB1,135 NET markers,136 and leukocyte-platelet aggregates.137
In mice, hematopoietic cell TF contributes to the coagulopathy observed models of endotoxemia.138 Infusions of extracellular histones in mice mimic the pathophysiology of DIC by inducing thrombocytopenia, the formation of platelet-rich microthrombi,83 and microvascular thrombosis, with concomitant bleeding.57 Additionally, elastase/cathepsin G-deficient mice demonstrated decreased fibrin deposition in response to Escherichia coli infection, and treatment with an anti-H2A-H2B-DNA antibody diminished fibrin production and microvascular occlusions.35 Similarly, infusion of exogenous HMGB1 can potentiate thrombosis and hemorrhage in kidney and lung microvasculature in a thrombin-induced model of DIC.10
Resolution of thrombosis by leukocytes
In addition to influencing thrombus induction, leukocytes regulate thrombus persistence and levels of activated or acute phase coagulation factors in the circulation. This includes modulation of fibrinolysis through expression and activation of fibrinolytic mediators. Additionally, leukocytes regulate thrombus resolution and coagulation factor clearance through phagocytosis.
Fibrinolysis
Both monocytes and neutrophils can modulate activity of the fibrinoytic pathway and susceptibility of formed fibrin to fibrinolysis (Figure 1). In vitro fibrinolysis is accelerated in the presence of isolated quiescent neutrophils139 and monocytes,140 and monocyte-derived microparticles141 through several characterized mechanisms. Leukocytes express uPA and its receptor uPAR,142 and hematopoietic uPA deficiency is associated with attenuated thrombus resolution in vivo.143 Leukocytes also express receptors for plasminogen, including enolase, Annexin II, and histone H2B, which localize plasminogen to the leukocyte surface, thereby enhancing activation by tissue-type plasminogen activator (tPA) and/or uPA.144 Additionally, elastase has been shown to inactivate plasminogen activator inhibitor145 and activate plasmin in the absence of tPA/uPA.146
However, under pathological circumstances, activated leukocytes may attenuate endogenous fibrinolytic mechanisms. Lipopolysaccharide-stimulated monocytes inhibit fibrinolysis by increasing activation of thrombin activatable fibrinolysis inhibitor in a TF-dependent manner.147 NETosis may also influence fibrinolysis and thrombus stability, as the addition of CF-DNA and histones to clotting plasma results in the formation of thicker fibers with greater mechanical stability.148 In patients with sepsis and elevated CF-DNA levels, clot lysis times are attenuated compared with controls149 ; this effect can be replicated by the addition of histone-DNA complex148 or in the presence of NETing neutrophils.53 The presence of CF-DNA within the clot has been shown to impair plasminogen activation by tPA,53 and the binding of plasmin to fibrin.150
Phagocytosis
In addition to modulation of fibrinolysis, phagocytic leukocytes play a vital role in regulating the persistence of active coagulation factors and formed thrombi within the vasculature. Plasma levels of coagulation factors strongly influence the propensity for pathological thrombus formation, and regulation of plasma levels of the coagulation factors involves a dynamic balance between biosynthesis, secretion, and clearance. Monocyte and/or macrophages express scavenger receptors, including low-density lipoprotein receptor family (eg, low density lipoprotein receptor-related protein 1), sialic acid-binding immunoglobulin-type lectin family members , and αMβ2 integrin, which regulate the endocytosis of coagulation factors such as VWF and FVIII,151,152 activated platelets,153 fibrin(ogen),154 and/or NETs.108,155 For example, the depletion of macrophages in vivo is associated with elevated plasma levels of VWF-FVIII,151 a risk-factor for venous and arterial thrombosis. Neutrophils can also contribute to the clearance of activated platelets through interactions mediated by platelet surface P-selectin binding to neutrophil-expressed PSGL-1. This interaction is stabilized by neutrophil-expressed β2 integrins, and requires phosphatidylserine exposure on the platelet surface to mediate endocytosis of the active platelet.156
Leukocytes also mediate venous thrombus resolution, a process that in addition to fibrinolysis and phagocytosis involves angiogenesis, fibrosis, and vessel wall remodeling.157,158 Circulating leukocytes are recruited to the thrombus by release of proinflammatory cytokines and chemokines, upregulated adhesion molecules on the endothelium,159 the binding of plasminogen to plasminogen receptors, and the formation of fibrin.160-162 Leukocyte infiltration is temporally regulated with neutrophils predominating at early stages, and monocytes and macrophages predominating at later stages.159 In animal models involving neutrophil depletion, IVC ligation in rats was associated with delayed thrombus resolution,157 although this effect is not observed in mice. However, impaired recruitment of monocytes to the resolving thrombus is associated with increased thrombus size, and decreased neovascularization in murine IVC stasis models.158,163
Although the role of neutrophils in thrombus resolution is not fully characterized, they may contribute to this process by phagocytosing apoptotic cells and by-products of thrombolysis. Monocytes have been shown to regulate thrombus resolution by influencing fibrinolysis, producing growth factors, matrix metalloproteinases,158 and uPA,164 which in addition to activating plasminogen mediates cell migration and tissue remodeling.143 Importantly, there may be heterogeneous roles for monocyte subtypes in the process of thrombus resolution. Ly6C+ monocytes, considered to be proinflammatory, are recruited early to damaged tissue, and have been hypothesized to play a role in phagocytosing apoptotic cells and debris associated with the thrombus. Conversely, Ly6C− resident monocytes patrol the vasculature, are recruited later to sites of vascular damage, and have been hypothesized to contribute to tissue repair.165,166
Active coagulation factors modulate leukocyte activity
Although leukocyte activation can modify blood coagulation, it is well recognized that active coagulation factors and platelets can also regulate the proinflammatory activity of leukocytes (Figure 4). In the context of thrombosis, this relationship of reciprocal activation can serve to recruit leukocytes to the forming thrombus. Both monocytes and macrophages express protease activated receptor-1 (PAR-1), a G-protein coupled receptor that is activated by coagulation factor proteases.167 Thrombin can induce chemotaxis of both neutrophils168 and monocytes169 via PAR-dependent and -independent mechanisms.170 Conversely, APC inhibits leukocyte chemotaxis through PAR cleavage, and interactions with β1 and β3 integrins.171,172 Active coagulation factors can also mediate the release of procoagulant materials and inflammatory agents by leukocytes. Thrombin can regulate the production of proinflammatory cytokines including interleukin-6 and tumor necrosis factor-α by monocytes through PAR signaling.173 Fibrin and fibrin degradation products can stimulate the release of proinflammatory cytokines from monocytes and macrophages by signaling through TLR4.160,161 Soluble fibrinogen is a potent inducer of neutrophil degranulation via interactions with αMβ2 integrin, and can increase phagocytic activity while delaying neutrophil apoptosis.162 Additionally, activated platelets can interact with neutrophils to induce degranulation156 and NET formation.41 Thus, positive feedback between dysregulated procoagulant and proinflammatory pathways within the developing thrombus may enhance the procoagulant phenotype of thrombus-associated leukocytes and exacerbate the development of pathological thrombosis.
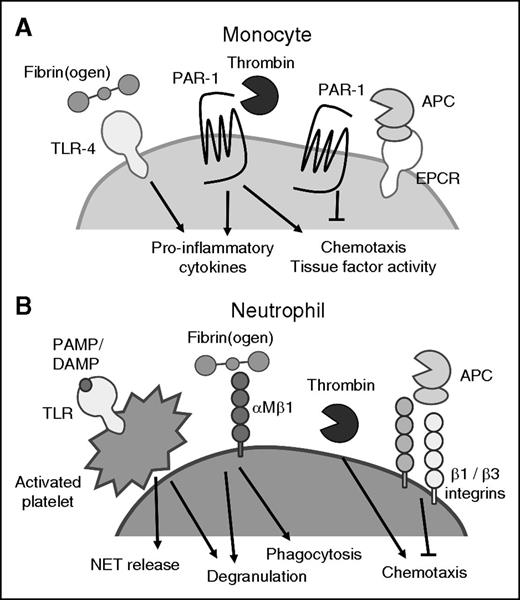
Coagulation factors can activate leukocytes. (A) Thrombin and APC cleave PAR-1 expressed on monocytes, and regulate monocyte proinflammatory and procoagulant properties. (B) Coagulation factors and activated platelets interact with neutrophils to regulate neutrophil degranulation, NET release, and phagocytic and chemotactic activities. APC, activated protein C; PAMP, pathogen-associated molecular pattern.
Coagulation factors can activate leukocytes. (A) Thrombin and APC cleave PAR-1 expressed on monocytes, and regulate monocyte proinflammatory and procoagulant properties. (B) Coagulation factors and activated platelets interact with neutrophils to regulate neutrophil degranulation, NET release, and phagocytic and chemotactic activities. APC, activated protein C; PAMP, pathogen-associated molecular pattern.
Inhibition of leukocyte procoagulant activity as antithrombotic therapy
Current strategies for the clinical management of thrombosis involve the use of prophylactic or on-demand anticoagulant therapies, which are associated with an increased risk for bleeding. Recognition of the contribution of leukocytes to the generation of pathological thrombosis, coupled with limited evidence that leukocytes participate in physiological hemostasis, has resulted in the recent development of rational strategies that specifically target leukocyte-mediated prothrombotic pathways in clinical and preclinical studies. Anti-inflammatory agents such as roflumilast (a phosphodiesterase-4 inhibitor) impair the recruitment of leukocytes to the site of thrombus formation,174 and are associated with a decreased risk for major cardiovascular events in chronic obstructive pulmonary disease patients.175 Additionally, statins, which have pleiotropic anticoagulant effects, including reduced expression of TF by monocytes and attenuated release of TF-expressing monocyte-derived microparticles in animal models of hypercholesterolemia,113 are associated with a decreased incidence of arterial and venous thrombosis in clinical studies.176,177
Preclinical studies that targeted leukocyte recruitment to the thrombus using monoclonal antibodies to P-selectin murine and primate models demonstrated reduced inflammation and/or venous thrombus formation.178,179 Other strategies have involved inhibiting the procoagulant agents released by activated leukocytes. Treatment with the PAD inhibitor Cl-amidine is capable of blocking NET release, and attenuates thrombus formation and reduces atherosclerotic lesion areas in murine models.123,124 Therapies that decrease the procoagulant activity of histones, including APC,57 recombinant soluble TM,180 non-anticoagulant heparins,181 and neutralizing antibodies,57 protect mice from thrombosis and/or histone-mediated death in animal models of acute inflammation. Dismantling NETs with DNAse is protective from flow-restricted venous thrombosis,103 and arterial thrombosis induced by photochemical injury in a murine model of chronic inflammation.123 Additionally, the utility of DNAse in combination with tPA for thrombolysis has demonstrated efficacy in ex vivo models.102 Nucleic acid-binding polymers, which inhibit nucleic acid- and polyphosphate-induced activation of the intrinsic pathway of coagulation have also been shown to prevent thrombosis in mice without increasing the risk of bleeding.182 Finally, targeting contact pathway activation, such as with monoclonal antibodies to FXII, can reduce thrombus formation in primate models183 and may mitigate some of the procoagulant effects associated with high levels of extracellular DNA.
Conclusions
Leukocytes are a dynamic and itinerant component of the innate immune system that mediate a rapid response to procoagulant stimuli. Localized intravascular coagulation involving activated leukocytes likely evolved as an adaptive mechanism to promote the resolution of infection when trauma and epidemics accounted for the majority of human deaths. Improved sanitation, nutrition, and treatment of infection promoted epidemiological transition, associated with an increased life expectancy, and the development of chronic inflammatory diseases such as atherosclerosis. Thus, the activation of leukocyte procoagulant activity in response to sterile inflammation may be maladaptive, and links the coincidence of micro- and macrovascular thrombosis with inflammatory pathologies.
There remain controversies in the literature that pertain to the heterarchical role neutrophils and monocytes play in thrombus formation that may be related to the model of thrombosis used, as well as the pathobiological context in which the thrombus develops. The continued development of physiologically relevant in vivo models of thrombosis, and careful assessment of the contribution that leukocytes make to thrombo-inflammatory conditions will provide novel insights into the mechanistic basis of thrombosis. These discoveries may be translated to the clinic through improved identification of individuals at-risk for thrombosis. They may also result in the discovery of novel targets for the development of prophylactic or on-demand anticoagulant treatment, and generate strategies for accelerating thrombus resolution. Although modulation of the axis between inflammation and coagulation must take into consideration the potentially beneficial role that leukocyte activities may have in regulating disease outcome and promotion of thrombus resolution, the development of novel inhibitors of leukocyte-associated procoagulant activity may ultimately prove effective at reducing the burden of thrombosis worldwide.
Acknowledgments
The authors thank T. Gould and L. Pepler for many helpful discussions.
L.L.S. is supported by a postdoctoral fellowship from the Canadian Institutes for Health Research, and P.C.L. is supported by a grant from the Canadian Institutes for Health Research (MOP-136878).
Authorship
Contribution: L.L.S. created the figures and wrote the manuscript, and P.C.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patricia C. Liaw, Thrombosis and Atherosclerosis Research Institute, McMaster University, 237 Barton St East, Room C5-107, Hamilton, ON L8L 2X2, Canada; e-mail: patricia.liaw@taari.ca.