Key Points
Response to the CD38-targeting antibody daratumumab is significantly associated with CD38 expression levels on the tumor cells.
Resistance to daratumumab is accompanied by increased expression of complement-inhibitory proteins.
Abstract
The anti-CD38 monoclonal antibody daratumumab is well tolerated and has high single agent activity in heavily pretreated relapsed and refractory multiple myeloma (MM). However, not all patients respond, and many patients eventually develop progressive disease to daratumumab monotherapy. We therefore examined whether pretreatment expression levels of CD38 and complement-inhibitory proteins (CIPs) are associated with response and whether changes in expression of these proteins contribute to development of resistance. In a cohort of 102 patients treated with daratumumab monotherapy (16 mg/kg), we found that pretreatment levels of CD38 expression on MM cells were significantly higher in patients who achieved at least partial response (PR) compared with patients who achieved less than PR. However, cell surface expression of the CIPs, CD46, CD55, and CD59, was not associated with clinical response. In addition, CD38 expression was reduced in both bone marrow–localized and circulating MM cells, following the first daratumumab infusion. CD38 expression levels on MM cells increased again following daratumumab discontinuation. In contrast, CD55 and CD59 levels were significantly increased on MM cells only at the time of progression. All-trans retinoic acid increased CD38 levels and decreased CD55 and CD59 expression on MM cells from patients who developed daratumumab resistance, to approximately pretreatment values. This resulted in significant enhancement of daratumumab-mediated complement-dependent cytotoxicity. Together, these data demonstrate an important role for CD38 and CIP expression levels in daratumumab sensitivity and suggest that therapeutic combinations that alter CD38 and CIP expression levels should be investigated in the treatment of MM. These trials were registered at www.clinicaltrials.gov as #NCT00574288 (GEN501) and #NCT01985126 (SIRIUS).
Introduction
In the last decade, survival of multiple myeloma (MM) patients has markedly improved.1 However, patients with disease refractory to immunomodulatory drugs (IMiDs) and proteasome inhibitors have a median overall survival of only 9 months,2 underscoring the need for additional active agents with novel mechanisms of action.3,4 Antibodies against target antigens expressed on MM cells are an important new class of agents, and preliminary results are very promising in this group of patients.5
The anti-CD38 monoclonal antibody, daratumumab, is well tolerated and has high single agent activity. In the 16 mg/kg cohort of the GEN501 study, at least a partial response (PR) was achieved in 36% of the patients including complete response in 5%.6 Similar efficacy was observed in the SIRIUS study.7 Based on these results, the US Food and Drug Administration recently approved daratumumab for the treatment of MM patients who have received ≥3 prior lines of therapy including a proteasome inhibitor and an IMiD or who are double refractory to a proteasome inhibitor and an IMiD. The mechanisms implicated in daratumumab-mediated killing of tumor cells include a direct apoptotic effect8 and, more important, activation of potent cytotoxic immune effector functions, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity (CDC).9-13 In fact, daratumumab was selected for further development because of its high efficacy to kill tumor cells via CDC.11 Moreover, myeloid-derived suppressor cells, regulatory B cells, and a subset of regulatory T cells also express CD38 and are susceptible to daratumumab-mediated lysis.14 It has also been shown that daratumumab modulates the enzymatic activity of CD38, which potentially leads to a reduction in immunosuppressive adenosine levels.15,16 This shift away from an immunosuppressive environment is hypothesized to result in an improved host–antitumor immune response.14
Despite the well-established clinical efficacy of daratumumab, not all of the heavily pretreated patients respond to single agent daratumumab, and the majority of patients who initially respond eventually progress. This indicates the need for new insights into mechanisms of resistance. We have previously shown that expression of CD38 on MM cells correlates with daratumumab-mediated ADCC and CDC in vitro.17 However, the predictive value of CD38 expression on MM cells for achieving clinical benefit from daratumumab is currently unknown. In addition, not all the variability in daratumumab-mediated killing in vitro could be explained by differences in CD38 expression. To further understand CD38-independent tumor-related mechanisms influencing daratumumab sensitivity, we investigated expression of complement-inhibitory proteins on MM cells.
Host cells are protected against accidental complement attack by fluid-phase regulators and by expression of membrane-associated complement-inhibitory proteins, such as CD46 and the glycosyl-phospatidylinositol-anchored proteins, CD55 and CD59.18,19 Overexpression of these complement-inhibitory proteins in cancer plays a role in tumor immune evasion and resistance against therapeutic antibodies.20-28
Here, we demonstrate that daratumumab-treated patients, who achieve at least PR, have higher CD38 baseline levels compared with patients with less than PR. No differences in pretreatment expression levels of the complement-inhibitory proteins were observed between both groups of patients. Furthermore, we show that development of resistance toward daratumumab was associated with upregulation of CD55 and CD59 on the MM cells. In addition, reduced surface expression of CD38 on nondepleted MM cells may also confer protection against daratumumab. Finally, we demonstrate that all-trans retinoic acid (ATRA) increases CD38 and reduces CD55 and CD59 expression on daratumumab-resistant MM cells, thereby improving CDC against MM cells.
Materials and methods
Patients and protocols
Data on expression levels of CD38 and complement-inhibitory proteins on bone marrow (BM)-localized MM cells were derived from patients with relapsed or refractory multiple myeloma treated with 16 mg/kg daratumumab monotherapy and who were enrolled in 2 clinical studies (NCT00574288 [GEN501] and NCT01985126 [SIRIUS]) that have been described in detail elsewhere.7,29
Briefly, in the GEN501 study, patients had MM requiring systemic therapy and relapsed from or refractory to ≥2 prior therapies.29 In the SIRIUS study, patients had received ≥3 prior lines of therapy including a proteasome inhibitor and an IMiD, or were refractory to both classes of drugs.7 In both studies patients had age ≥18 years; life expectancy ≥3 months; Eastern Cooperative Oncology Group performance status of ≤2; and measurable disease. Exclusion criteria included other malignancies; uncontrolled infections; cardiovascular and respiratory conditions; or meningeal involvement of MM.
Before start of daratumumab therapy, BM aspirates were obtained from 102 patients treated in the GEN501 and SIRIUS studies. In addition, in a subset of 21 patients, treated in the GEN501 study, BM aspirates were also obtained ∼14 weeks after initiation of treatment and at the time of progression. In this group of patients, peripheral blood was also obtained before start, at multiple time points during treatment, and after administration of the last daratumumab infusion.
Study site ethics committees or institutional review boards approved the protocols, which were conducted according to the principles of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients gave written informed consent.
Antibodies and reagents
Flow cytometric analysis of BM and blood samples from patients treated with daratumumab monotherapy
CD38, CD46, CD55, and CD59 expression levels were determined on both BM-localized and circulating MM cells by using flow cytometric analysis.
Additional methods are presented in the supplemental Data, available on the Blood Web site.
Results
CD38 expression levels on pretreatment MM cells are associated with clinical response to daratumumab
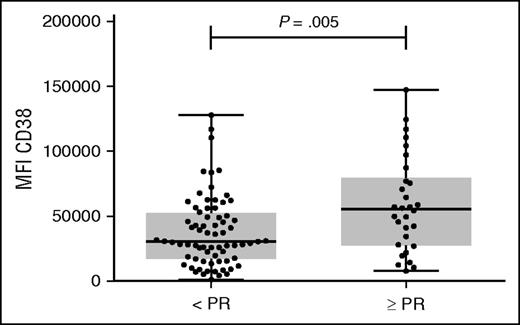
We have previously shown that there is a significant positive association between CD38 expression levels on MM cells from patients and the efficacy of daratumumab to induce cell death by ADCC, as well as CDC.17 We hypothesized that cell surface expression of CD38 on MM cells may predict response to daratumumab monotherapy. We therefore analyzed CD38 expression on pretreatment BM-localized MM cells from patients, who were subsequently treated with 16 mg/kg daratumumab as single agent in the phase 2 part of the GEN501 study29 or in the SIRIUS study.7 Pretreatment BM samples for this analysis were available for 102 of a total of 148 MM patients treated with 16 mg/kg daratumumab. The clinical characteristics of these heavily pretreated patients are shown in Table 1. As expected, all MM cells expressed CD38 antigen in these patients’ samples, but there was a marked heterogeneity in the intensity of CD38 expression. In this group of 102 patients, at least PR was achieved in 30 patients (29%). The MM patients who achieved at least PR had significantly higher baseline CD38 expression levels on their tumor cells compared with patients who achieved less than PR (median fluorescence intensity (MFI) CD38: 55 424 vs 30 659; P = .005; Figure 1). Accordingly, response to daratumumab was markedly higher in the highest tertile of CD38 expression (≥PR: 48.5%) compared with the mid-tertile (22.2%) or lowest tertile (18.2%).
Patient characteristics
| Parameter . | MM patients (n = 102) . |
|---|---|
| Median age, years (range) | 64 (32-84) |
| Sex, male, n (%) | 57 (56%) |
| M-protein type | |
| IgG, n (%) | 51 (50%) |
| IgA, n (%) | 21 (21%) |
| IgM, n (%) | 0 (0%) |
| IgD, n (%) | 3 (3%) |
| Biclonal, n (%) | 2 (2%) |
| Light chain only, n (%) | 25 (25%) |
| Previous therapy | |
| ≤3 lines of therapy | 24 (24%) |
| >3 lines of therapy | 78 (76%) |
| Prior therapy to which disease was refractory*before start of daratumumab | |
| Lenalidomide refractory, n (%) | 86 (84%) |
| Bortezomib refractory, n (%) | 85 (83%) |
| Lenalidomide and bortezomib refractory, n (%) | 78 (76%) |
| Pomalidomide refractory, n (%) | 54 (53%) |
| Carfilzomib refractory, n (%) | 36 (35%) |
| Parameter . | MM patients (n = 102) . |
|---|---|
| Median age, years (range) | 64 (32-84) |
| Sex, male, n (%) | 57 (56%) |
| M-protein type | |
| IgG, n (%) | 51 (50%) |
| IgA, n (%) | 21 (21%) |
| IgM, n (%) | 0 (0%) |
| IgD, n (%) | 3 (3%) |
| Biclonal, n (%) | 2 (2%) |
| Light chain only, n (%) | 25 (25%) |
| Previous therapy | |
| ≤3 lines of therapy | 24 (24%) |
| >3 lines of therapy | 78 (76%) |
| Prior therapy to which disease was refractory*before start of daratumumab | |
| Lenalidomide refractory, n (%) | 86 (84%) |
| Bortezomib refractory, n (%) | 85 (83%) |
| Lenalidomide and bortezomib refractory, n (%) | 78 (76%) |
| Pomalidomide refractory, n (%) | 54 (53%) |
| Carfilzomib refractory, n (%) | 36 (35%) |
Refractory disease is defined as progressive disease during therapy, no response (less than PR), or progressive disease within 60 days of stopping treatment, according to the International Uniform Response Criteria for Multiple Myeloma.
CD38 expression levels on primary MM cells before start of therapy are significantly higher in responders vs. nonresponders in GEN501 and SIRIUS studies. CD38 expression levels were analyzed by flow cytometry in 102 BM samples obtained from relapsed/refractory MM patients before start of treatment with daratumumab monotherapy at a dose of 16 mg/kg in the GEN501 and SIRIUS studies. HuMax-003 FITC was used as CD38 antibody. Shown are median, 25th-75th percentile (box), and minimum and maximum value (whiskers). CD38 expression levels on MM cells were compared between responders (defined as partial response or better) and nonresponders. P values between the indicated groups were calculated using the Mann-Whitney U test. Every dot represents a patient. N, number.
CD38 expression levels on primary MM cells before start of therapy are significantly higher in responders vs. nonresponders in GEN501 and SIRIUS studies. CD38 expression levels were analyzed by flow cytometry in 102 BM samples obtained from relapsed/refractory MM patients before start of treatment with daratumumab monotherapy at a dose of 16 mg/kg in the GEN501 and SIRIUS studies. HuMax-003 FITC was used as CD38 antibody. Shown are median, 25th-75th percentile (box), and minimum and maximum value (whiskers). CD38 expression levels on MM cells were compared between responders (defined as partial response or better) and nonresponders. P values between the indicated groups were calculated using the Mann-Whitney U test. Every dot represents a patient. N, number.
Importantly, CD38 levels on MM cells were similar in patients with or without double-refractory (lenalidomide and bortezomib-refractory), triple-refractory (lenalidomide, bortezomib, and either pomalidomide or carfilzomib-refractory), or quadruple refractory disease (lenalidomide, pomalidomide, bortezomib, and carfilzomib-refractory). Also age, sex, tumor load, creatinine clearance, lenalidomide treatment before daratumumab, lactate dehydrogenase levels, β2-microglobulin levels, and International Staging System stage did not affect CD38 expression. Soluble CD38, which may also bind daratumumab, was evaluated in 110 of the 148 patients and detected in only 2 cases. Both of them achieved PR.
Expression levels of complement-inhibitory proteins on pretreatment MM cells are not associated with daratumumab-mediated CDC or clinical response to daratumumab
Given the overlap in CD38 expression levels between responding and nonresponding patients, we concluded that CD38 levels alone do not explain the whole variability in response to daratumumab therapy. Because daratumumab has high CDC activity,11 which explains the consumption of complement proteins C2 and C4 (and to a lesser extent C3, but not C1q) after the first daratumumab infusion (supplemental Figure 1), we hypothesized that cell surface expression of the complement-inhibitory proteins CD46, CD55, and CD59 on MM cells could be associated with the extent of daratumumab-mediated CDC, as well as response to therapy. Therefore, we evaluated the impact of complement-inhibitory proteins on daratumumab’s ability to kill tumor cells by using cell lines, primary MM cells, and also pretreatment samples from patients treated in the GEN501 and SIRIUS studies.
We first analyzed 14 MM and 19 non-Hodgkin’s lymphoma cell lines in CDC assays. Twenty-seven cell lines were completely resistant, and 6 cell lines were sensitive to daratumumab-mediated CDC (Figure 2A). The sensitive cell lines had higher CD38 (P = .0019) and lower CD59 (P = .0006) expression levels compared with the resistant ones. In addition, there was a trend toward higher levels of CD55 expression in CDC-resistant cell lines, whereas elevated CD46 levels were not associated with daratumumab resistance. The role of CD55 and CD59 as possible determinants of susceptibility toward daratumumab was further investigated by pretreating cells with phospholipase-C, which removed the glycosylphosphatidylinositol-anchored CD55 and CD59 molecules from the cell surface but had no effect on CD38 expression (Figure 2B). Pretreatment with phospholipase-C rendered 7 of 14 cell lines more susceptible to complement-mediated lysis (Figure 2C). The cell lines without improved CDC after pretreatment with phospholipase-C had significantly lower CD38 expression compared with the 7 cell lines that showed enhanced CDC (P = .02).
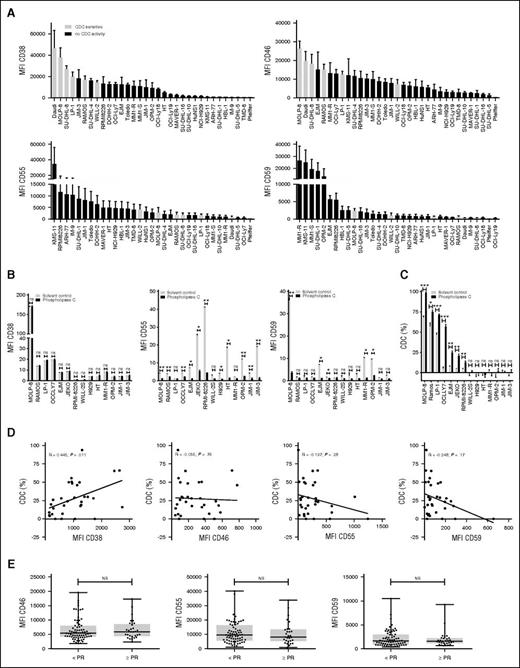
Expression of complement-inhibitory proteins and susceptibility to daratumumab-mediated CDC. (A) Susceptibility of 33 MM and lymphoma cell lines toward daratumumab-mediated CDC according to expression levels of CD38, CD46, CD55, and CD59 as determined by flow cytometry. Daratumumab induced CDC in 6 of 33 cell lines (white bars), whereas the others were completely resistant (black bars). Shown are mean ± SEM of 6 experiments. (B) Expression levels of CD38, CD55, and CD59 on 14 MM and lymphoma cell lines, after incubation with solvent control (white bar) or phospholipase-C (black bar) for 30 minutes. Shown are mean ± SEM. P values between the indicated groups were calculated using paired Student t tests. (C) CDC assays were performed with the same 14 cell lines as shown in B, which were pretreated with phospholipase-C (black bars) or solvent control (white bars) for 30 minutes. CDC assays were performed with 10 μg/mL daratumumab or IgG1-b12 control antibody as described in Materials and methods. Shown are mean ± SEM of 3 experiments. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ***P < .001, ns, not significant. (D) Positive correlation between CD38 expression levels on primary MM cells and CDC mediated by 10 μg/mL daratumumab at 1 hour (n = 32 MM patients). No significant correlation between CD55, CD59, and CD46 expression levels on primary MM cells and CDC mediated by 10 μg/mL daratumumab at 1 hour (n = 32 MM patients). Expression levels were determined by flow cytometry. CDC assays were performed as described in Materials and methods. Correlations between variables were analyzed using the Spearman’s rank correlation coefficient. (E) CD46, CD55, and CD59 expression levels were analyzed by flow cytometry in 102 BM samples obtained from relapsed/refractory MM patients before start of treatment with daratumumab monotherapy at a dose of 16 mg/kg in the GEN501 and SIRIUS studies. Shown are median, 25th-75th percentile (box), and minimum and maximum value (whiskers). CD46, CD55, and CD59 expression levels on MM cells were compared between responders (defined as PR or better) and nonresponders. P values between the indicated groups were calculated using the Mann-Whitney U test. Every dot represents a patient.
Expression of complement-inhibitory proteins and susceptibility to daratumumab-mediated CDC. (A) Susceptibility of 33 MM and lymphoma cell lines toward daratumumab-mediated CDC according to expression levels of CD38, CD46, CD55, and CD59 as determined by flow cytometry. Daratumumab induced CDC in 6 of 33 cell lines (white bars), whereas the others were completely resistant (black bars). Shown are mean ± SEM of 6 experiments. (B) Expression levels of CD38, CD55, and CD59 on 14 MM and lymphoma cell lines, after incubation with solvent control (white bar) or phospholipase-C (black bar) for 30 minutes. Shown are mean ± SEM. P values between the indicated groups were calculated using paired Student t tests. (C) CDC assays were performed with the same 14 cell lines as shown in B, which were pretreated with phospholipase-C (black bars) or solvent control (white bars) for 30 minutes. CDC assays were performed with 10 μg/mL daratumumab or IgG1-b12 control antibody as described in Materials and methods. Shown are mean ± SEM of 3 experiments. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ***P < .001, ns, not significant. (D) Positive correlation between CD38 expression levels on primary MM cells and CDC mediated by 10 μg/mL daratumumab at 1 hour (n = 32 MM patients). No significant correlation between CD55, CD59, and CD46 expression levels on primary MM cells and CDC mediated by 10 μg/mL daratumumab at 1 hour (n = 32 MM patients). Expression levels were determined by flow cytometry. CDC assays were performed as described in Materials and methods. Correlations between variables were analyzed using the Spearman’s rank correlation coefficient. (E) CD46, CD55, and CD59 expression levels were analyzed by flow cytometry in 102 BM samples obtained from relapsed/refractory MM patients before start of treatment with daratumumab monotherapy at a dose of 16 mg/kg in the GEN501 and SIRIUS studies. Shown are median, 25th-75th percentile (box), and minimum and maximum value (whiskers). CD46, CD55, and CD59 expression levels on MM cells were compared between responders (defined as PR or better) and nonresponders. P values between the indicated groups were calculated using the Mann-Whitney U test. Every dot represents a patient.
Next we examined the impact of complement-inhibitory proteins on daratumumab-mediated CDC by using BM samples from 32 daratumumab-naive MM patients. Twenty-one of these 32 patients were subsequently treated with daratumumab monotherapy in the GEN501 study. The characteristics of these patients are shown in supplemental Table 1. Daratumumab (10 µg/mL)-mediated CDC against MM cells was very heterogeneous and ranged from −15.5% (negative values indicate MM cell growth) to 93.9% (median, 22.5%). There was a positive correlation between susceptibility of primary MM cells to CDC and the level of CD38 (R = 0.45; P = .011), which is in agreement with our previous analysis in a different cohort of patients.17 However, expression levels of CD46, CD55, and CD59 were not correlated with CDC (Figure 2D).
We also analyzed complement inhibitor expression on pretreatment MM cells from the 102 patients with available baseline BM samples, who subsequently received daratumumab treatment in the GEN501 and SIRIUS studies (Figure 2E). There was no significant difference in baseline expression levels of CD46, CD55, and CD59 between patients who achieved at least PR compared with patients with less than PR. We also tested whether the combined biologic effects of CD38 and complement inhibitors were associated with response by calculating the ratios of CD38/CD46, CD38/CD55, and CD38/CD59. However, there was no improvement in the strength of the association with response compared with CD38 expression levels alone (data not shown). Therefore, to gain a more detailed insight on the role of these molecules during treatment, we next performed an in-depth longitudinal analysis of CD38 and complement-inhibitory proteins in a subset of 21 GEN501 patients.
Reduced CD38 expression on MM cells during daratumumab treatment
Because CD38 expression on MM cells is an important determinant of susceptibility toward daratumumab,17 we hypothesized that residual daratumumab-resistant MM cells may have decreased levels of this protein. To this end, we analyzed CD38 expression on BM-localized MM cells in a subset of GEN501 patients (n = 21) before start of treatment, 14 weeks after the initiation of daratumumab treatment, and also at the time of progression during daratumumab therapy. We used an anti-CD38 monoclonal antibody, HuMax-003-fluorescein isothiocyanate (FITC), that binds to a different epitope compared with daratumumab. This excluded the possibility that binding of daratumumab masked the detection of CD38.
Interestingly, 14 weeks after the first daratumumab infusion, the MM cells had significantly lower CD38 expression levels compared with baseline values (median MFI CD38: 866.0 vs 124.2, P = .0001; Figure 3A-B). Similarly, at the time of progression MM cells had low CD38 expression levels (median MFI CD38: 85.1). The reduced expression of CD38 is a transient phenomenon because ∼6 months after the last daratumumab infusion BM-localized MM cells regained CD38 expression (Figure 3A). There was no difference between patients who did or did not achieve PR or better and decrease in CD38 expression during daratumumab treatment (Figure 3C).
Daratumumab treatment is associated with decreased levels of CD38 on MM cells. (A) CD38 expression on MM cells in BM samples obtained from 21 patients, who were subsequently treated with daratumumab at a dose of 16 mg/kg in the GEN501 study. BM aspirates were obtained before start of daratumumab, before the 10th daratumumab infusion, at the time of progression (PD), and 6 months after stopping daratumumab therapy because of progressive disease (PD+6M). CD38 expression was determined by using HuMax-003-FITC, which binds to a different epitope compared with daratumumab, thereby excluding the possibility that binding of daratumumab masked the detection of CD38. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; **P < .01, ***P < .001, ****P < .0001, ns, not significant. (B) Flow cytometry histogram overlays depicting cell surface expression of CD38 on MM cells from 4 representative patients treated with daratumumab in the GEN501 trial at different time points: before start of treatment (green histogram), during daratumumab treatment before the 10th infusion (blue histogram), and at the time of progressive disease (red histogram). HuMax-003 FITC was used as CD38 antibody. (C) Longitudinal data representation of CD38 expression levels on MM cells from the 21 patients presented in A, according to the response achieved to daratumumab monotherapy (PR or better [gray bars] vs less than partial response [black bars]). P values between the indicated groups were calculated using a Student t test; **P < .01, ***P < .001, ****P < .0001. (D) Longitudinal data representation of absolute circulating MM cell counts over time in peripheral blood. Circulating MM cells were observed in 11 of 21 patients tested. Peripheral blood was obtained before start of treatment with daratumumab, during treatment with daratumumab, at the time of progression (PD), as well as 2, 4, and 6 months after development of progressive disease (PD). Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05. (E) CD38 expression on circulating MM cells before start of daratumumab treatment, during daratumumab treatment, at the time of progression (PD), as well as 2, 4, and 6 months after the development of progressive disease (PD) (n = 11 patients). CD38 expression was determined by using HuMax-003-FITC. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ***P < .001, ns, not significant.
Daratumumab treatment is associated with decreased levels of CD38 on MM cells. (A) CD38 expression on MM cells in BM samples obtained from 21 patients, who were subsequently treated with daratumumab at a dose of 16 mg/kg in the GEN501 study. BM aspirates were obtained before start of daratumumab, before the 10th daratumumab infusion, at the time of progression (PD), and 6 months after stopping daratumumab therapy because of progressive disease (PD+6M). CD38 expression was determined by using HuMax-003-FITC, which binds to a different epitope compared with daratumumab, thereby excluding the possibility that binding of daratumumab masked the detection of CD38. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; **P < .01, ***P < .001, ****P < .0001, ns, not significant. (B) Flow cytometry histogram overlays depicting cell surface expression of CD38 on MM cells from 4 representative patients treated with daratumumab in the GEN501 trial at different time points: before start of treatment (green histogram), during daratumumab treatment before the 10th infusion (blue histogram), and at the time of progressive disease (red histogram). HuMax-003 FITC was used as CD38 antibody. (C) Longitudinal data representation of CD38 expression levels on MM cells from the 21 patients presented in A, according to the response achieved to daratumumab monotherapy (PR or better [gray bars] vs less than partial response [black bars]). P values between the indicated groups were calculated using a Student t test; **P < .01, ***P < .001, ****P < .0001. (D) Longitudinal data representation of absolute circulating MM cell counts over time in peripheral blood. Circulating MM cells were observed in 11 of 21 patients tested. Peripheral blood was obtained before start of treatment with daratumumab, during treatment with daratumumab, at the time of progression (PD), as well as 2, 4, and 6 months after development of progressive disease (PD). Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05. (E) CD38 expression on circulating MM cells before start of daratumumab treatment, during daratumumab treatment, at the time of progression (PD), as well as 2, 4, and 6 months after the development of progressive disease (PD) (n = 11 patients). CD38 expression was determined by using HuMax-003-FITC. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ***P < .001, ns, not significant.
To gain further insight into the kinetics of CD38 reduction, we analyzed CD38 expression on circulating MM cells in the same GEN501 subgroup. Peripheral blood clonal plasma cells could be detected before start and during daratumumab therapy in 11 of 21 patients (52%). Circulating MM cells were rapidly cleared by daratumumab (Figure 3D). Already after the first daratumumab infusion the nondepleted circulating MM cells had significantly lower CD38 expression levels compared with baseline (P = .0006; Figure 3E). Examination of peripheral blood samples, taken at different time points after disease progression, showed that CD38 expression gradually increased to baseline levels ∼6 months after daratumumab treatment. Also on the circulating MM cells, there was no difference in the reduction of CD38 levels during daratumumab treatment between patients who achieved PR and those with less than PR (supplemental Figure 2).
Increased CD55 and CD59 levels on MM cells at the time of progression during daratumumab therapy
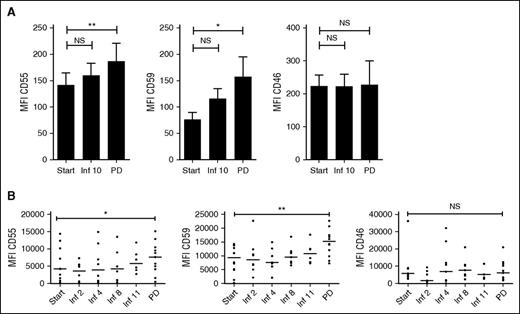
To further analyze mechanisms of resistance to daratumumab, we analyzed expression of the complement-inhibitory proteins on BM-localized MM cells in the GEN501 subgroup (n = 21). There was no change in expression of the complement-inhibitory proteins when patients were still responding to daratumumab or had stable disease. However, there was a significant increase in CD55 and CD59 expression levels on BM-localized MM cells at the time of progression compared with levels before start or during daratumumab treatment (median MFI CD55: 109.0 vs 167.0, P = .01; median MFI CD59: 50.3 vs 98.2, P = .018 for samples taken at baseline and at the time of progression, respectively; Figure 4A). Importantly, CD46 protein levels did not increase at the time of progression.
Expression levels of CD55 and CD59 on MM cells increase at the time of progression. (A) CD55, CD59, and CD46 expression levels on MM cells from BM samples obtained from 21 MM patients, who were subsequently treated with daratumumab at a dose of 16 mg/kg in the GEN501 study. BM samples were obtained before start of daratumumab, before the 10th daratumumab infusion, and at the time of progression (PD). Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ns, not significant. (B) CD55, CD59, and CD46 expression levels on circulating MM cells before start of daratumumab treatment, during daratumumab treatment, and at the time of progression (PD) (n = 11 patients). Expression levels of the complement-inhibitory proteins were analyzed by flow cytometry. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ns, not significant.
Expression levels of CD55 and CD59 on MM cells increase at the time of progression. (A) CD55, CD59, and CD46 expression levels on MM cells from BM samples obtained from 21 MM patients, who were subsequently treated with daratumumab at a dose of 16 mg/kg in the GEN501 study. BM samples were obtained before start of daratumumab, before the 10th daratumumab infusion, and at the time of progression (PD). Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ns, not significant. (B) CD55, CD59, and CD46 expression levels on circulating MM cells before start of daratumumab treatment, during daratumumab treatment, and at the time of progression (PD) (n = 11 patients). Expression levels of the complement-inhibitory proteins were analyzed by flow cytometry. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ns, not significant.
Similarly, on circulating MM cells, CD55 and CD59 levels did not change until patients developed progressive disease (increase in median MFI CD55 of 81.5%, P = .031; increase in median MFI CD59 of 63.3%, P = .0049, for samples taken at baseline and at the time of progression). CD46 expression on circulating MM cells remained unchanged (Figure 4B).
Daratumumab treatment selects for MM clones with high expression levels of CD55 and CD59
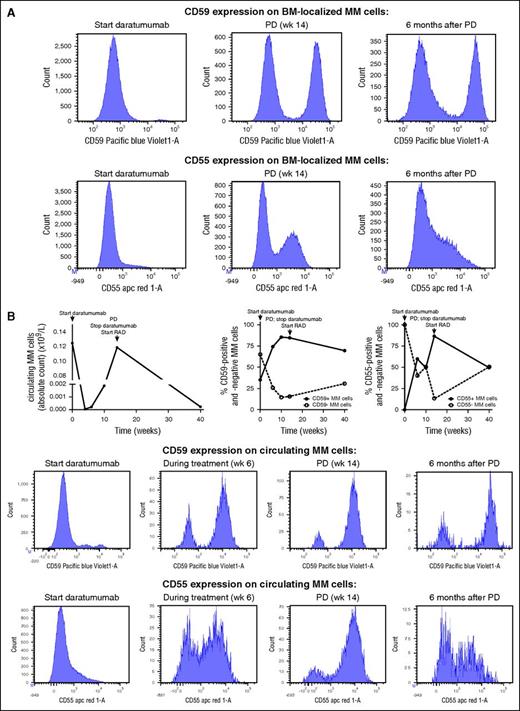
There is increasing evidence of existence of competing subclones in MM.32-36 We therefore investigated the presence of phenotypic subclones based on CD38 and complement-inhibitory protein expression levels. In the majority of patients, 1 phenotypic population of MM cells was detected based on expression of CD38 and complement inhibitors. However, in 2 of 21 patients, we detected 2 coexisting populations of MM cells based on differential expression of CD55 and/or CD59. To investigate the effect of daratumumab on the relative frequencies of these different subpopulations, we analyzed several blood and/or BM samples from these patients before the start of treatment, during daratumumab treatment, at the time of progression, and also during additional follow-up. In both patients, we observed a change in subclone phenotypes with rapid selection of daratumumab-resistant clones with high expression of complement-inhibitors (Figure 5 [patient 5]; supplemental Figure 3 [patient 6]). CD38 expression levels were similar in the different MM cell populations from patient 5. However, baseline CD38 expression levels were lower in CD59-positive cells from patient 6 compared with the CD59-negative cells, which may have contributed to the selection of the CD59-positive subpopulation.
Selection of populations of MM cells with high expression of complement-inhibitory proteins during therapy with daratumumab. Two of 21 GEN501 patients had coexisting populations of MM cells, which differed in CD55 and/or CD59 expression. (A) BM-localized MM cells from patient 5 differed in expression levels of CD55 (absent or strongly positive) and CD59 (absent or strongly positive). Bone marrow samples were obtained before start of daratumumab therapy, at the time of progressive disease (PD), and 6 months after the last daratumumab infusion. Flow cytometry histograms for these samples are shown. (B) Similarly, circulating MM cells from patient 5 also differed in expression levels of CD55 (absent or strongly positive) and CD59 (absent or strongly positive). Serial blood samples were obtained during daratumumab monotherapy and during the treatment given after development of progressive disease (PD). Longitudinal data representation of absolute circulating MM cell counts over time and of the frequency of the different subpopulations based on complement-inhibitory protein expression. Representative histograms are also shown for this patient. RAD, lenalidomide, adriamycin, and dexamethasone.
Selection of populations of MM cells with high expression of complement-inhibitory proteins during therapy with daratumumab. Two of 21 GEN501 patients had coexisting populations of MM cells, which differed in CD55 and/or CD59 expression. (A) BM-localized MM cells from patient 5 differed in expression levels of CD55 (absent or strongly positive) and CD59 (absent or strongly positive). Bone marrow samples were obtained before start of daratumumab therapy, at the time of progressive disease (PD), and 6 months after the last daratumumab infusion. Flow cytometry histograms for these samples are shown. (B) Similarly, circulating MM cells from patient 5 also differed in expression levels of CD55 (absent or strongly positive) and CD59 (absent or strongly positive). Serial blood samples were obtained during daratumumab monotherapy and during the treatment given after development of progressive disease (PD). Longitudinal data representation of absolute circulating MM cell counts over time and of the frequency of the different subpopulations based on complement-inhibitory protein expression. Representative histograms are also shown for this patient. RAD, lenalidomide, adriamycin, and dexamethasone.
ATRA reverses resistance to daratumumab by increasing CD38 expression levels
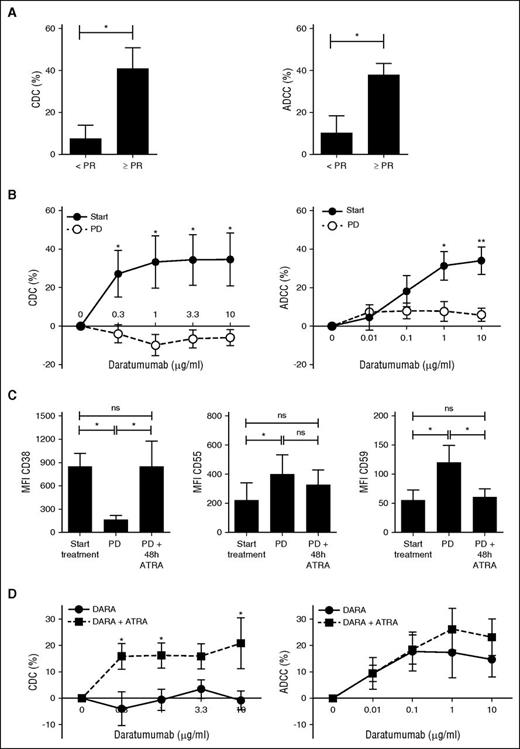
From 15 of the previously described 21 GEN501 patients, we harvested sufficient BM–mononuclear cells (MNCs) with which we performed ex vivo CDC and ADCC assays. Daratumumab-mediated CDC and ADCC in these pretreatment samples was associated with clinical response to daratumumab therapy (Figure 6A). In serial samples (n = 5), there was a marked reduction in daratumumab-mediated CDC and ADCC against MM cells from aspirates taken at the time of progression compared with paired pretreatment samples (CDC with 10 µg/mL daratumumab: 34.6% to −6.0%, P = .02; ADCC with 10 µg/mL daratumumab: 34.1-5.9%, P = .007; Figure 6B). Thus, the results from these ex vivo experiments are consistent with the daratumumab-resistant phenotype of the MM cells in the patients at the time of progression. Importantly, daratumumab that is possibly bound to MM cells after initiation of daratumumab treatment had no CDC activity, because the IgG1-b12 control antibody in the presence of native human serum did not induce lysis of MM cells (lysis: −1.14%).
ATRA reverses resistance to daratumumab-mediated CDC by increasing CD38 expression and reducing expression levels of complement-inhibitory proteins on MM cells. (A) Ex vivo CDC and ADCC assays were performed with BM aspirates from 15 patients, who were subsequently treated in the GEN501 study. One-hour CDC and 48-hour ADCC assays were performed as described in Materials and methods. Shown are means ± SEM of CDC and ADCC mediated by 10 µg/mL daratumumab according to response achieved in the GEN501 study. P values between the indicated groups were calculated using a Student t test; *P < .05. (B) Serial BM aspirates were obtained from 5 patients both before start of daratumumab and at the time of progression during daratumumab. One-hour CDC and 48-hour ADCC assays were performed as described in Materials and methods with pretreatment samples and samples obtained at the time of progression. We expect that at the time of progression MM cells have already daratumumab, given to the patient via intravenous infusion, bound to their cell surface; therefore, these graphs show the effect of freshly added daratumumab. To evaluate the effect of possible prebound daratumumab in CDC assays, we analyzed the effect of the control antibody IgG1-b12 (no CDC activity). IgG1-b12 in the presence of heat-inactivated serum or native human serum did not induce CDC against MM cells obtained at the time of progression (lysis: 0.81% and −1.14%). This indicates that prebound daratumumab does also not induce CDC at the time of progression. Dose-response curves for ADCC and CDC were constructed according to treatment status. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01. (C) BM-MNCs were obtained from 8 patients before the first daratumumab infusion and at the time of progression (PD). BM-MNCs obtained at the time of progression were subsequently incubated with solvent control or with 10 nM ATRA for 48 hours. Cells were then collected to determine CD38, CD55, and CD59 expression levels by flow cytometry. HuMax-003-FITC was used to detect CD38 expression. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, ns, not significant. (D) Pooled results of 1-hour CDC and 48-hour ADCC assays, using BM-MNCs of 6 patients. BM-MNCs were pretreated for 48 hours with solvent control or 10 nM ATRA, followed by incubation with IgG1-b12 control antibody or daratumumab. Pooled human serum (10%) was used as a source of complement. The survival of primary CD138+ MM cells in the BM-MNCs was determined by flow cytometry. Percentage lysis of MM cells was calculated as indicated in Materials and methods. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05.
ATRA reverses resistance to daratumumab-mediated CDC by increasing CD38 expression and reducing expression levels of complement-inhibitory proteins on MM cells. (A) Ex vivo CDC and ADCC assays were performed with BM aspirates from 15 patients, who were subsequently treated in the GEN501 study. One-hour CDC and 48-hour ADCC assays were performed as described in Materials and methods. Shown are means ± SEM of CDC and ADCC mediated by 10 µg/mL daratumumab according to response achieved in the GEN501 study. P values between the indicated groups were calculated using a Student t test; *P < .05. (B) Serial BM aspirates were obtained from 5 patients both before start of daratumumab and at the time of progression during daratumumab. One-hour CDC and 48-hour ADCC assays were performed as described in Materials and methods with pretreatment samples and samples obtained at the time of progression. We expect that at the time of progression MM cells have already daratumumab, given to the patient via intravenous infusion, bound to their cell surface; therefore, these graphs show the effect of freshly added daratumumab. To evaluate the effect of possible prebound daratumumab in CDC assays, we analyzed the effect of the control antibody IgG1-b12 (no CDC activity). IgG1-b12 in the presence of heat-inactivated serum or native human serum did not induce CDC against MM cells obtained at the time of progression (lysis: 0.81% and −1.14%). This indicates that prebound daratumumab does also not induce CDC at the time of progression. Dose-response curves for ADCC and CDC were constructed according to treatment status. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01. (C) BM-MNCs were obtained from 8 patients before the first daratumumab infusion and at the time of progression (PD). BM-MNCs obtained at the time of progression were subsequently incubated with solvent control or with 10 nM ATRA for 48 hours. Cells were then collected to determine CD38, CD55, and CD59 expression levels by flow cytometry. HuMax-003-FITC was used to detect CD38 expression. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, ns, not significant. (D) Pooled results of 1-hour CDC and 48-hour ADCC assays, using BM-MNCs of 6 patients. BM-MNCs were pretreated for 48 hours with solvent control or 10 nM ATRA, followed by incubation with IgG1-b12 control antibody or daratumumab. Pooled human serum (10%) was used as a source of complement. The survival of primary CD138+ MM cells in the BM-MNCs was determined by flow cytometry. Percentage lysis of MM cells was calculated as indicated in Materials and methods. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05.
We have previously shown that ATRA increases CD38 expression levels and reduces CD55 and CD59 levels on MM cells. This resulted in enhanced ADCC and CDC in both MM cell lines and patient samples.17 However, these MM cells were not previously exposed to daratumumab. Because our data suggest that reduced CD38 expression and increased CD55 and CD59 levels contribute to acquired resistance to daratumumab, we hypothesized that ATRA may also be of value at the time of progression. We first tested whether ATRA could restore CD38 expression and reduce CD55 and CD59 levels on daratumumab-resistant MM cells. To this end, we incubated BM-MNCs, obtained from 8 MM patients with progressive disease during daratumumab therapy, with ATRA. This resulted in a significant upregulation of CD38 expression, but also in a reduction of CD55 and CD59 levels on the MM cells, almost to pretreatment values (Figure 6C).
Next, we treated BM-MNCs obtained from patients with progressive disease during daratumumab therapy with solvent control or ATRA for 48 hours, followed by incubation with or without daratumumab. ATRA alone for 48 or 96 hours did not significantly affect MM cell viability compared with solvent control. However, pretreatment with ATRA for 48 hours improved daratumumab-mediated CDC in 4 of 6 patients and ADCC in 2 of 6 patients. Pooled results show that ATRA pretreatment improved daratumumab (10 μg/mL)-mediated CDC from −0.8% to 20.9% (P = .031) and ADCC from 14.8 to 23.2% (P = .31; Figure 6D).
Discussion
Treatment with single agent daratumumab is clinically effective in relapsed and refractory MM, although there is a fraction of these heavily pretreated MM patients that does not respond to daratumumab. Furthermore, the majority of responding patients will develop resistance over time. Mechanisms that influence daratumumab efficacy are probably multifactorial including both host- and tumor-related factors. This study combined prospective clinical data with correlative measurements on blood and BM specimens to evaluate the impact of several tumor-related factors on response and development of resistance to daratumumab monotherapy.
The current study shows that CD38 expression on MM cells is associated with response to daratumumab therapy. Several other clinical studies have also shown that efficacy of monoclonal antibodies including rituximab,37,38 alemtuzumab,39 and trastuzumab40,41 is partly dependent on target antigen expression. Importantly, it is unlikely that CD38 is a general prognostic factor in MM. First, CD38 gene expression levels were not associated with response in the Hovon-65/GMMG-HD4 study, in which patients did not receive daratumumab (data not shown). Moreover, we found that CD38 expression was not affected by prior therapies or by markers of aggressive disease. Therefore, our data indicate that CD38 is a predictor of response to daratumumab.
However, the variability in clinical outcome following daratumumab treatment cannot solely be explained by differential expression of CD38, which precludes its use as a definitive predictive biomarker of response to daratumumab in clinical practice. It is likely that other tumor-related factors such as genetic abnormalities and activation status of signaling pathways, as well as differences in the composition of the BM microenvironment including frequency of immune effector and suppressor cells, also contribute to the variability in response to daratumumab. Furthermore, extent of target saturation is an important determinant of response, with 16 mg/kg daratumumab as the lowest tested dose with pharmacokinetics that are consistent with target saturation.29 Because daratumumab has potent CDC activity, we also evaluated expression levels of the complement inhibitory proteins CD46, CD55, and CD59. Although, reduction of CD55 and CD59 expression improved daratumumab-mediated CDC in cell lines with substantial CD38 expression, expression levels of complement-inhibitory proteins were not associated with response in MM patients treated with daratumumab monotherapy.
However, analysis of serial blood and BM samples revealed that CD55 and CD59 levels were increased on MM cells at the time of progression compared with baseline values. Importantly, ex vivo experiments showed that at the time of progression MM cells were resistant to daratumumab-mediated killing. Altogether, this suggests that CD55 and CD59 protein levels increase during the acquisition of a resistant phenotype. Similarly, CD55 and CD59 expression levels are not correlated with susceptibility to rituximab-mediated CDC and do not predict clinical outcome in lymphoma and CLL patients treated with rituximab.25,42-44 However, there is an increase in CD55 and CD59 levels on CLL cells that were not cleared from blood by rituximab therapy.43 Interestingly, there is also a positive correlation between naturally occurring anti-MUC1 antibody levels and expression of the complement-inhibitory proteins CD46, CD55, and CD59 in patients with bladder cancer.28 Altogether, this indicates that complement-inhibitory proteins may be a broad resistance mechanism for monoclonal antibodies that function through CDC.
Furthermore, we show that daratumumab treatment resulted in a rapid reduction of CD38 levels on both BM-localized and circulating MM cells. There are several possible explanations for the reduction of CD38 levels on MM cells. First, in responding patients, daratumumab may select for tumor cells with lower CD38 expression while preferentially eliminating MM cells with high CD38 levels. In addition, downregulation of CD38 may be an active process to evade daratumumab-mediated killing. Furthermore, recent in vitro studies suggest that binding of daratumumab to CD38 may cause redistribution of CD38 molecules, formation of distinct polar aggregates, and subsequent release of tumor microvesicles.16 Finally, trogocytosis of CD38-daratumumab complexes by Fcγ receptor-expressing effector cells and direct internalization may also play a role in loss of CD38.45 Downregulation of CD38 on MM cells is a transient event, because ∼6 months after the last daratumumab infusion, CD38 expression increases again. This may be explained by persisting circulating daratumumab during this period resulting in continuous selective pressure. Daratumumab concentrations in serum were not determined after administration of the last infusion. However, interference of daratumumab in the indirect antiglobulin test, as a result of binding to CD38-positive donor erythrocytes, persisted 2 to 6 months after the last daratumumab infusion,46 indicating that daratumumab remains present in serum for up to 6 months. Similarly, measurable circulating rituximab can persist for up to 6 months after treatment.47 Importantly, because CD38 levels return to baseline values ∼6 months after the last daratumumab infusion, retreatment with daratumumab may be effective and warrants further investigation. Similarly, recent studies demonstrated substantial and rapid reduction of CD20 in CLL48-51 and lymphoma52 patients treated with rituximab or ofatumumab,53 which has been linked to development of acquired resistance.48,51-54
Although the rapid loss of CD38 may allow MM cells to escape from daratumumab-mediated killing, CD38 reduction was observed in patients with both <PR and ≥PR, including those with sustained clinical response, which raises the possibility that the continuous pressure to maintain MM cells in a CD38−/low state offers a clinical benefit in the treatment of CD38-positive malignancies. Physiologic ligands for CD38 and CD31 are present on BM stromal cells and endothelial cells, as well as hyaluronic acid, which is an extracellular matrix component. It has recently been demonstrated that overexpression of CD38 on MM cells results in increased adherence to BM stromal cells probably via CD38–CD31 interactions.55 Reduced expression of CD38 may therefore lead to loss of cell–cell and cell–matrix contacts, which may contribute to reduced MM cell growth and survival. In addition, CD38 also functions as an ectoenzyme and in this role it has been implicated in immune suppression through production of adenosine in the BM microenvironment.56,57 Daratumumab-mediated reduction of CD38 on MM cells may therefore contribute to an improved host–antitumor immune response.14
By using immunophenotypic analysis, we demonstrated in 2 patients the presence of unique subpopulations with different expression levels of complement inhibitors, whose relative frequencies changed during daratumumab therapy and also after stopping this treatment. This dynamic picture of back and forth competition between phenotypic subclones during and after daratumumab is similar to what has been previously demonstrated with the use of fluorescence in situ hybridization, array comparative genomic hybridization, and whole-exome/genome sequencing.32-35 Our data support the importance of intraclonal heterogeneity in MM with multiple clones having a different clinical behavior and differential sensitivity to treatment.36
Modulation of determinants of daratumumab sensitivity with novel therapeutic approaches may lead to more effective daratumumab-based regimens with increased quality of response and improved survival. As CD38 levels are significantly reduced following daratumumab treatment and CD38 expression determines susceptibility to daratumumab, we hypothesized that upregulation of CD38 expression levels would lead to resensitization of daratumumab-resistant MM cells. We have previously shown that ATRA increases CD38 expression on daratumumab-naive MM cells.17 In this study, we demonstrated that ATRA restored expression of CD38, but also reduced CD55 and CD59 levels to close to preinfusion levels, on daratumumab-resistant MM cells from patients with progressive disease. This resulted in significant enhancement of CDC and a modest improvement in ADCC. This differential effect can be explained by the fact that ATRA-mediated reductions of CD55 and CD59 will improve CDC, but not ADCC. Furthermore, the moderate enhancement of ADCC may also be related to low frequencies of NK cells at the time of progression compared with baseline values (T.C., Xu Steven Xu, Homer Adams III, A.E.A., Bie Verbist, Kevin Liu, Imran Khan, Tahamtan Ahmadi, Xiaoyu Yuan, Sagar Lonial, Torben Plesner, H.M.L., N.W.C.J.v.d.D., Pamela L. Clemens, and A.K.S., unpublished data, June 1, 2016), without ATRA affecting NK cell levels or their activity.17
In conclusion, this study demonstrates that CD38 expression is associated with response to daratumumab monotherapy. We also showed that the development of daratumumab resistance may occur by acquisition of a new drug-induced phenotype with higher CD55 and CD59 expression levels or as a result of the emergence of a preexisting subpopulation that is already relatively resistant to daratumumab prior to initiation of therapy. We also provide the rationale for retreatment with daratumumab after sufficient time to allow CD38 expression levels to return to baseline on remaining MM cells or by adding ATRA to daratumumab regimens. These hypotheses should be explored in upcoming clinical studies with daratumumab.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the GEN501 and SIRIUS studies and their families, as well as the study coinvestigators, research nurses, and coordinators at each of the clinical sites.
The clinical studies were supported by research funding from Janssen Research and Development and Genmab, and the analyses presented here were supported by research funding by Janssen Research and Development.
Authorship
Contribution: I.S.N., T.C., J.v.V., B.v.K., A.E.A., M.v.D., and K.S. executed the experiments and analyzed and interpreted the results; A.K.S. provided daratumumab; H.M.L., M.C.M., I.S.N., S.Z., and N.W.C.J.v.d.D. provided patient materials; T.M., H.M.L., R.W.J.G., A.C.B., C.C., P.S., A.K.S., I.S.N., and N.W.C.J.v.d.D. designed the study and interpreted the results; I.S.N. and N.W.C.J.v.d.D. wrote the first draft of the manuscript; and all authors helped critically review the manuscript and checked the final version of it.
Conflict-of-interest disclosure: T.C., A.E.A., K.S., C.C., and A.K.S. are employees of Janssen Research and Development. H.M.L., T.M., R.W.J.G., and N.W.C.J.v.d.D. received research support from Janssen Research and Development. The remaining authors declare no competing financial interests.
Correspondence: Niels W. C. J. van de Donk, Department of Hematology, VU University Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: n.vandedonk@vumc.nl.

![Figure 3. Daratumumab treatment is associated with decreased levels of CD38 on MM cells. (A) CD38 expression on MM cells in BM samples obtained from 21 patients, who were subsequently treated with daratumumab at a dose of 16 mg/kg in the GEN501 study. BM aspirates were obtained before start of daratumumab, before the 10th daratumumab infusion, at the time of progression (PD), and 6 months after stopping daratumumab therapy because of progressive disease (PD+6M). CD38 expression was determined by using HuMax-003-FITC, which binds to a different epitope compared with daratumumab, thereby excluding the possibility that binding of daratumumab masked the detection of CD38. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; **P < .01, ***P < .001, ****P < .0001, ns, not significant. (B) Flow cytometry histogram overlays depicting cell surface expression of CD38 on MM cells from 4 representative patients treated with daratumumab in the GEN501 trial at different time points: before start of treatment (green histogram), during daratumumab treatment before the 10th infusion (blue histogram), and at the time of progressive disease (red histogram). HuMax-003 FITC was used as CD38 antibody. (C) Longitudinal data representation of CD38 expression levels on MM cells from the 21 patients presented in A, according to the response achieved to daratumumab monotherapy (PR or better [gray bars] vs less than partial response [black bars]). P values between the indicated groups were calculated using a Student t test; **P < .01, ***P < .001, ****P < .0001. (D) Longitudinal data representation of absolute circulating MM cell counts over time in peripheral blood. Circulating MM cells were observed in 11 of 21 patients tested. Peripheral blood was obtained before start of treatment with daratumumab, during treatment with daratumumab, at the time of progression (PD), as well as 2, 4, and 6 months after development of progressive disease (PD). Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05. (E) CD38 expression on circulating MM cells before start of daratumumab treatment, during daratumumab treatment, at the time of progression (PD), as well as 2, 4, and 6 months after the development of progressive disease (PD) (n = 11 patients). CD38 expression was determined by using HuMax-003-FITC. Data are presented as mean ± SEM. P values between the indicated groups were calculated using a paired Student t test; *P < .05, **P < .01, ***P < .001, ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/7/10.1182_blood-2016-03-703439/6/m_959f3.jpeg?Expires=1770977683&Signature=y4s2u9xNNFAJ5jXoNHo3x5Atym8X3cUqS6hubvaulUe2~-Yb9jkHsQ3bVO4bDx1HaONvBfS4gQbz8M2uGA7ErOaSerxxCYd4zSI~oVZO~YQGWrcsYuPC-iQqvqOE6E0hwEvqt7OC12~qEwXGOnbqXcsktxgcyNXrxH5VNzNa2QKv9ZF4calFt4mjQ814F~xA5ifHzDZrWaENfs~RcnaGi9apahQBBP-wFjtK-0opC5bceb2IB7cpxR9a-65DMIcS2zFKGmg3kX06ZPB94aWIqBi0AtDKtvNtfZ4n9Z-d1ii0X2LUGi6NB1qMTCKvHbPf~VQjjfOGkwqaG-44xEyYmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)