Key Points
JAK3-mediated phosphorylation of EZH2 resulted in EZH2 oncogenic function independent of its enzymatic activity.
Targeted inhibition of JAK3 may be a promising treatment in NK/TL through suppressing noncanonical EZH2 activity.
Abstract
The best-understood mechanism by which EZH2 exerts its oncogenic function is through polycomb repressive complex 2 (PRC2)-mediated gene repression, which requires its histone methyltransferase activity. However, small-molecule inhibitors of EZH2 that selectively target its enzymatic activity turn out to be potent only for lymphoma cells with EZH2-activating mutation. Intriguingly, recent discoveries, including ours, have placed EZH2 into the category of transcriptional coactivators and thus raised the possibility of noncanonical signaling pathways. However, it remains unclear how EZH2 switches to this catalytic independent function. In the current study, using natural killer/T-cell lymphoma (NKTL) as a disease model, we found that phosphorylation of EZH2 by JAK3 promotes the dissociation of the PRC2 complex leading to decreased global H3K27me3 levels, while it switches EZH2 to a transcriptional activator, conferring higher proliferative capacity of the affected cells. Gene expression data analysis also suggests that the noncanonical function of EZH2 as a transcriptional activator upregulates a set of genes involved in DNA replication, cell cycle, biosynthesis, stemness, and invasiveness. Consistently, JAK3 inhibitor was able to significantly reduce the growth of NKTL cells, in an EZH2 phosphorylation-dependent manner, whereas various compounds recently developed to inhibit EZH2 methyltransferase activity have no such effect. Thus, pharmacological inhibition of JAK3 activity may provide a promising treatment option for NKTL through the novel mechanism of suppressing noncanonical EZH2 activity.
Introduction
EZH2 is a H3K27-specific histone methyltransferase and a component of the polycomb repressive complex 2 (PRC2), which plays a key role in the epigenetic maintenance of repressive chromatin mark. EZH2 protein must partner with other noncatalytic proteins, such as EED and SUZ12, to form the PRC2 in order to carry out its histone methyltransferase activity. It is well established that EZH2 plays an important role in tumorigenesis, and its overexpression is associated with invasive growth and poor clinical outcomes in hematologic as well as epithelial cancers. The best-understood mechanism by which EZH2 exerts its oncogenic function is to act as a transcriptional repressor through its effect on chromatin via its histone methyltransferase activity; thus, inhibitors of EZH2 have been developed to target EZH2 enzymatic activity or EZH2-PRC2 functions in malignancies.1,2 Although B-cell lymphomas carrying a EZH2-activating mutation are highly sensitive to these compounds, no convincing data have emerged showing antitumor activity of catalytic EZH2 inhibitors in other cancers, including solid tumors where EZH2 is mainly overexpressed but not mutated. Interestingly, recent discoveries, including ours, have shown that EZH2 could switch to a “transcriptional activator” function and act independent of the PRC2 complex to drive cancer progression.3-7 We believe that this noncanonical function of EZH2 will be crucial in a number of malignancies and warrant further studies.
Understanding the mechanisms controlling EZH2 noncanonical function in tumor cells is critical for developing more effective therapeutic strategies. We have recently identified an oncogenic role for EZH2 independent of its methyltransferase activity in natural killer/T-cell lymphoma (NKTL) pathogenesis, where EZH2 directly activates gene transcription conferring its oncogenic potential. An important question raised is how EZH2 switches to its noncanonical function. JAK3 is a nonreceptor tyrosine kinase that mediates signals initiated by cytokines and growth factor receptors. Recent data indicate that persistent activation of JAK3 due to gain-of-function mutations is involved in several hematologic malignancies, including acute megakaryoblastic leukemia, T-cell acute lymphoblastic leukemia, cutaneous T-cell lymphoma, mantle-cell lymphoma, Burkitt lymphoma, and NKTL. Strikingly, 35.4% cases of NKTL harbor such JAK3 activating mutations, which confer the growth advantage in NKTL.8,9 In this study, we exploited a possible functional interaction between EZH2 and JAK3 in NKTL where both molecules are highly oncogenic to induce similar proliferative phenotypes. We found that JAK3 induces the phosphorylation of EZH2 to establish the oncogenic function of EZH2 independent of its catalytic activity. Our findings provide a new mechanism to define the oncogenic activity of JAK3 and suggest that inhibition of JAK3-mediated EZH2 activity may provide a promising therapeutic approach for NKTL.
Materials and methods
For details, see the supplemental Materials and Methods, available on the Blood Web site.
Results
JAK3 interacts with EZH2 and reduces H3K27 trimethylation in malignant NK cells
To address whether EZH2 might be a target of JAK3, we first used coimmunoprecipitation assays to study their possible interactions. In NK-tumor cell lines, JAK3 was detected in EZH2 immunoprecipitates (Figure 1A; supplemental Figure 1A) and vice versa (Figure 1B; supplemental Figure 1B) in total cell lysates, suggesting a physiological association between endogenous EZH2 and JAK3. Interestingly, we also detected EZH2 and JAK3 protein interaction using the chromatin extract, which selectively isolates chromatin-bound factors (supplemental Figure 1C).
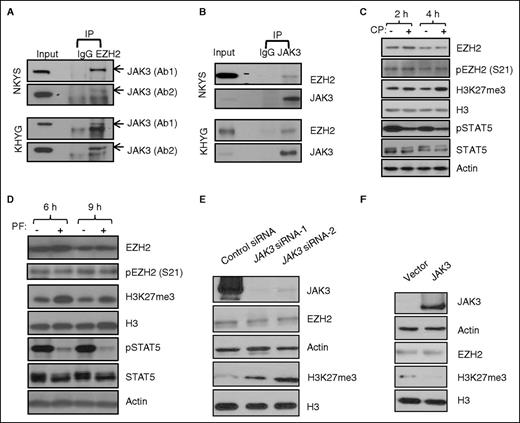
JAK3 physically interacts with EZH2 and negatively regulates H3K27 trimethylation. (A-B) Coimmunoprecipitation experiments to examine the interaction between endogenous EZH2 and JAK3 in NKYS or KHYG cell lines. Ab1 and Ab2 are 2 different JAK3 antibodies (Santa Cruz SC-513 and Cell Signaling 3775s for Ab1 and Ab2, respectively). (C) Immunoblot analysis of indicated proteins from NKYS cells treated with or without 0.5 μM CP-690550 (CP). (D) Immunoblot analysis of indicated proteins from NKYS cells treated with or without 1 μM PF956980 (PF). (E) Immunoblot analysis of H3 K27 trimethylation and EZH2 in NKYS cells transfected with control siRNA or 2 different individual JAK3 siRNAs for 24 hours. (F) Immunoblot analysis of H3 K27 trimethylation and EZH2 in 293T cells transfected with control vector or JAK3 expression plasmid for 48 hours.
JAK3 physically interacts with EZH2 and negatively regulates H3K27 trimethylation. (A-B) Coimmunoprecipitation experiments to examine the interaction between endogenous EZH2 and JAK3 in NKYS or KHYG cell lines. Ab1 and Ab2 are 2 different JAK3 antibodies (Santa Cruz SC-513 and Cell Signaling 3775s for Ab1 and Ab2, respectively). (C) Immunoblot analysis of indicated proteins from NKYS cells treated with or without 0.5 μM CP-690550 (CP). (D) Immunoblot analysis of indicated proteins from NKYS cells treated with or without 1 μM PF956980 (PF). (E) Immunoblot analysis of H3 K27 trimethylation and EZH2 in NKYS cells transfected with control siRNA or 2 different individual JAK3 siRNAs for 24 hours. (F) Immunoblot analysis of H3 K27 trimethylation and EZH2 in 293T cells transfected with control vector or JAK3 expression plasmid for 48 hours.
EZH2, the catalytic subunit of PRC2, is the main histone methyltransferase that methylates lysine 27 on histone H3. Given that JAK3 kinase interacts with EZH2 protein, this raises the possibility that alteration in JAK3 activity may change H3K27 trimethylation. Interleukin-2 (IL-2) in the culture medium is an essential cytokine required for the proliferation and activation of NK cells. It is known that IL-2 induces the activation of JAK/STAT5 signaling, which can be attenuated by a pan-JAK inhibitor, CP-690550,10,11 at 0.5 μM (Figure 1C). Interesting, reduction of STAT5 phosphorylation by CP-690550 treatment was accompanied with transiently increased H3K27 trimethylation (H3K27me3) (Figure 1C). Similarly, cells treated with another specific and chemically distinct JAK3 inhibitor PF95698012,13 for 6 hours also expressed enhanced H3K27me3 (Figure 1D). These results indicate that H3K27me3 is dynamically inhibited by JAK3 in NK tumor cells, suggesting a novel JAK3 function. To further establish the role of JAK3 in regulating H3K27me3, we showed that H3K27me3 was markedly increased when JAK3 was knocked down by 2 independent small interfering RNAs (siRNAs) (Figure 1E). Conversely, overexpression of JAK3 resulted in a reduced H3K27me3 (Figure 1F). Of notice, EZH2 expression at both mRNA (supplemental Figure 1D) and protein levels (Figure 1E-F) was not affected by JAK3, and neither was its subcellular localization in chromatin-bound and in soluble nuclear fractions (supplemental Figure 1E). Together, these observations raised a possibility that JAK3 might regulate H3K27me3 through posttranslational modification of the EZH2.
EZH2 Y244 site-specific phosphorylation mediates the effects of activated JAK3
As JAK3 is a tyrosine kinase, we postulated that JAK3-mediated phosphorylation of EZH2 at tyrosine residues may be involved in inhibiting EZH2 activity toward H3K27me3. Using antiphosphotyrosine antibody on immunoprecipitated EZH2 in NK cells, we found EZH2 to be phosphorylated on tyrosine (Figure 2A). This EZH2 phosphorylation is dependent of JAK3 as treatment of JAK3 inhibitor reduced tyrosine-phosphorylated EZH2, suggesting a role of JAK3 in the kinase-mediated modification of EZH2 protein (Figure 2B; supplemental Figure 2A).
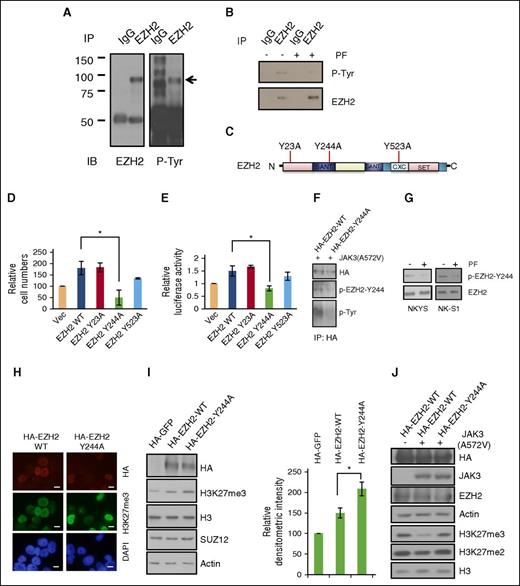
EZH2 Y244 site-specific phosphorylation mediates the effects of activated JAK3. (A) Coimmunoprecipitation assay showing that EZH2 is subject to tyrosine phosphorylation. NKYS cells were used to immunoprecipitate EZH2 and to probe antiphosphotyrosine antibody. (B) Coimmunoprecipitation assay showing that tyrosine phosphorylation of EZH2 in NKYS cells in the presence or absence of JAK3 inhibitor PF. EZH2 was immunoprecipitated from cells and were immunoblotted with antiphosphotyrosine antibodies to reveal the tyrosine phosphorylation levels of EZH2. (C) The top 3 phosphorylated tyr residues predicted by multiple phosphorylation site prediction programs. The 3 phosphorylation defective mutants were generated by mutating Y to A. (D) MTS cell viability assay showing different abilities of ectopic expression of EZH2 WT/mutants to affect cell growth of NKYS cells. Cells were transfected with the control empty vector pcDNA4.1 or EZH2 expression plasmids. The mean values of triplicate samples are shown, and error bars indicate standard deviation (SD). n = 4 independent experiments. *P < .05 (Student t test). (E) Luciferase reporter assay showing the effect of different EZH2 mutants on CCND1 promoter activity. NKYS cells were transfected with the luciferase reporter construct pGL4 containing the CCND1 promoter and various amounts of EZH2 WT/mutant plasmids or a control vector. Luciferase activities were measured after 24 hours. Luciferase readings were further normalized to the internal control pRL null. Results are presented as averages of triplicate experiments. Error bars indicate SD. *P < .05 (Student t test). (F) Coimmunoprecipitation assay of EZH2 tyrosine phosphorylation in 293T cells cotransfected with EZH2 WT/Y244A and JAK3 A572A for 48 hours. Transfected EZH2 was immunoprecipitated from cells using antihemagglutinin (HA) antibody and was immunoblotted with pEZH2-Y244 antibody or antiphosphotyrosine antibody to reveal the tyrosine phosphorylation level of EZH2. (G) Immunoblot analysis of pEZH2-Y244 levels in NKTL cells exposed to JAK3 inhibitor PF for 2 hours. (H) Immunofluorescent staining of H3 K27 trimethylation in NKYS cells transfected with EZH2 WT or Y244A expression plasmids for 2 days. Scale bars, 10 μm. The HA-EZH2-positive staining cells from EZH2 Y244A in general exhibit a stronger increase in the staining (relative to those nontransfected cells in the same field) for H3 K27 trimethylation compared with those positive staining cells from EZH2 WT. (I) Representative of immunoblot (left) and densitometric quantification (right; n = 3 independent experiments) of H3K27 trimethylation in 293T cells transfected with EZH2 WT/Y244A expression plasmids or control vector for 48 hours. Results are means ± SD. *P < .05 (Student t test). Expression of EZH2 WT and the Y244A mutant was detected by the HA-tag antibody. H3 and actin were used as loading controls. (J) Immunoblot analysis of H3 K27 trimethylation in 293T cells cotransfected with JAK3 A572V and EZH2 WT/Y244A expression plasmid or control vectors for 48 hours. Expression of EZH2 WT and the Y244A mutant was detected by the HA-tag antibody.
EZH2 Y244 site-specific phosphorylation mediates the effects of activated JAK3. (A) Coimmunoprecipitation assay showing that EZH2 is subject to tyrosine phosphorylation. NKYS cells were used to immunoprecipitate EZH2 and to probe antiphosphotyrosine antibody. (B) Coimmunoprecipitation assay showing that tyrosine phosphorylation of EZH2 in NKYS cells in the presence or absence of JAK3 inhibitor PF. EZH2 was immunoprecipitated from cells and were immunoblotted with antiphosphotyrosine antibodies to reveal the tyrosine phosphorylation levels of EZH2. (C) The top 3 phosphorylated tyr residues predicted by multiple phosphorylation site prediction programs. The 3 phosphorylation defective mutants were generated by mutating Y to A. (D) MTS cell viability assay showing different abilities of ectopic expression of EZH2 WT/mutants to affect cell growth of NKYS cells. Cells were transfected with the control empty vector pcDNA4.1 or EZH2 expression plasmids. The mean values of triplicate samples are shown, and error bars indicate standard deviation (SD). n = 4 independent experiments. *P < .05 (Student t test). (E) Luciferase reporter assay showing the effect of different EZH2 mutants on CCND1 promoter activity. NKYS cells were transfected with the luciferase reporter construct pGL4 containing the CCND1 promoter and various amounts of EZH2 WT/mutant plasmids or a control vector. Luciferase activities were measured after 24 hours. Luciferase readings were further normalized to the internal control pRL null. Results are presented as averages of triplicate experiments. Error bars indicate SD. *P < .05 (Student t test). (F) Coimmunoprecipitation assay of EZH2 tyrosine phosphorylation in 293T cells cotransfected with EZH2 WT/Y244A and JAK3 A572A for 48 hours. Transfected EZH2 was immunoprecipitated from cells using antihemagglutinin (HA) antibody and was immunoblotted with pEZH2-Y244 antibody or antiphosphotyrosine antibody to reveal the tyrosine phosphorylation level of EZH2. (G) Immunoblot analysis of pEZH2-Y244 levels in NKTL cells exposed to JAK3 inhibitor PF for 2 hours. (H) Immunofluorescent staining of H3 K27 trimethylation in NKYS cells transfected with EZH2 WT or Y244A expression plasmids for 2 days. Scale bars, 10 μm. The HA-EZH2-positive staining cells from EZH2 Y244A in general exhibit a stronger increase in the staining (relative to those nontransfected cells in the same field) for H3 K27 trimethylation compared with those positive staining cells from EZH2 WT. (I) Representative of immunoblot (left) and densitometric quantification (right; n = 3 independent experiments) of H3K27 trimethylation in 293T cells transfected with EZH2 WT/Y244A expression plasmids or control vector for 48 hours. Results are means ± SD. *P < .05 (Student t test). Expression of EZH2 WT and the Y244A mutant was detected by the HA-tag antibody. H3 and actin were used as loading controls. (J) Immunoblot analysis of H3 K27 trimethylation in 293T cells cotransfected with JAK3 A572V and EZH2 WT/Y244A expression plasmid or control vectors for 48 hours. Expression of EZH2 WT and the Y244A mutant was detected by the HA-tag antibody.
To identify potential sites in EZH2 that is phosphorylated by JAK3, we first used the phosphorylation site prediction programs (NetworKIN 3.0, GPS 3.0, DISPHOS 1.3, and NetPhos 2.0). The top 3 phosphorylated tyrosine residues predicted are Y23, Y244, and Y523; none of which has been reported (Figure 2C). To confirm the critical tyr-phosphorylation site on EZH2, we generated 3 phosphorylation defective mutants in which each of these tyrosine residues was substituted independently with alanine. Because EZH2 overexpression in NK cell line confers cell growth advantage,6 the substitution mutants of EZH2 were then tested for their ability to promote cell growth. 3-(4,5 Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay shows that NK cell line expressing the EZH2 Y244A mutant had significantly reduced cell growth, whereas the other 2 mutants had similar cell growth as NK cell lines expressing EZH2 wild type (WT) (Figure 2D). This result indicates that single-site substitution of EZH2 Y244 is sufficient to abolish its growth-inducing effects. Our previous study showed that EZH2 induced growth and proliferation of NK cells through transactivation of CCND1 (encoding cyclin D1) via a mechanism that is not dependent on its SET domain, crucial for EZH2 histone methyltransferase activity.6 We therefore checked whether the 3 mutants have the same capability as WT to upregulate the CCND1 promoter activity using luciferase reporter assay. Consistent with the MTS data, only the Y244A mutant induced significantly lower luciferase activity compared with vector control (Figure 2E). These results demonstrate that substitution of Y244 with alanine within EZH2 compromises EZH2 transcriptional activation of the CCND1 gene and affects cell growth. Therefore, we speculate that tyrosine 244 is probably a crucial site for EZH2 to exert its nonenzymatic function and its pro-proliferative effect on NKTL.
Next, we investigated whether JAK3 is able to phosphorylate EZH2 at the Y244 site. The Tyr 244 residue in EZH2, along with neighboring residues, is evolutionarily conserved from zebrafish to mammals (supplemental Figure 2B). We generated a rabbit polyclonal antibody that specifically recognizes EZH2 phosphorylation at Y244. We verified its specificity against EZH2 by immunoprecipitation (IP; supplemental Figure 2C) and confirmed its phosphospecificity by dot-blot assay using phospho- or nonphosphopeptides (supplemental Figure 2D). When we replaced the Y244 residue with alanine (EZH2-Y244A), ectopic expression of activated JAK3 was no longer able to phosphorylate the mutant EZH2 demonstrated by the Y244 phospho-EZH2 antibody (Figure 2F). Confirming this, the phosphorylation level of EZH2 Y244A mutant was almost undetectable in an immunoblot using antiphosphotyrosine antibody (Figure 2F). Importantly, treatment of NKTL cells with JAK3 inhibitor reduced the level of pEZH2-Y244 (Figure 2G). Thus, these results demonstrate that Y244 in EZH2 is indeed phosphorylated by JAK3. Next we asked whether the phosphorylation of Y244 is responsible for regulating H3K27me3 levels. Consistent with preceding data showing that JAK3 inhibition increases H3K27me3 levels (Figure 1), we observed that the H3K27me3 level was higher in cells transfected with plasmids encoding the mutant EZH2-Y244A than in cells transfected with plasmids encoding EZH2 WT (Figure 2H-I). Furthermore, JAK3-mediated downregulation of H3K27me3 level requires Y244 site-specific phosphorylation in EZH2 (Figure 2J). Similar to the results in NKYS (Figure 2D), the EZH2 Y244A mutant led to attenuated cell growth compared with EZH2 WT in 293T cells cotransfected with JAK3 A572V (supplemental Figure 2E). Collectively, these results demonstrate that Y244 site-specific phosphorylation in EZH2 mediates the effects of activated JAK3 in maintaining cell growth while downregulating H3K27me3.
EZH2 phosphorylation by JAK3 is inhibitory for PRC2-mediated gene repression but required for gene activation toward proliferation
To estimate whether phosphorylation of EZH2 by JAK3 has an impact on transcription of PRC2-repressed genes, which is associated with EZH2 canonical activity, we performed gene expression microarray on JAK3 siRNA-treated NKYS cells and analyzed the data using Gene Set Enrichment Analysis (GSEA). We found that the significantly enriched gene sets downregulated with JAK3 knockdown included H3K27me3, SUZ12, and canonical EZH2 target gene sets (Figure 3A), suggesting a restored epigenetic silencing effect on inhibition of EZH2 phosphorylation. To see if this is relevant in patients, we examined a gene expression dataset of patient samples. This dataset comprises samples from 19 unique NKTL patients, inclusive of 9 NKTL samples already previously published.14 We derived a JAK-STAT activation gene signature and a PRC2-repressed target gene signature (see “supplemental Materials and methods”). Indeed, our analysis revealed a strong positive correlation between JAK-STAT signature and the PRC2-repressed target gene signature (Figure 3B), suggesting that JAK3 activation weakens EZH2’s canonical silencing function.
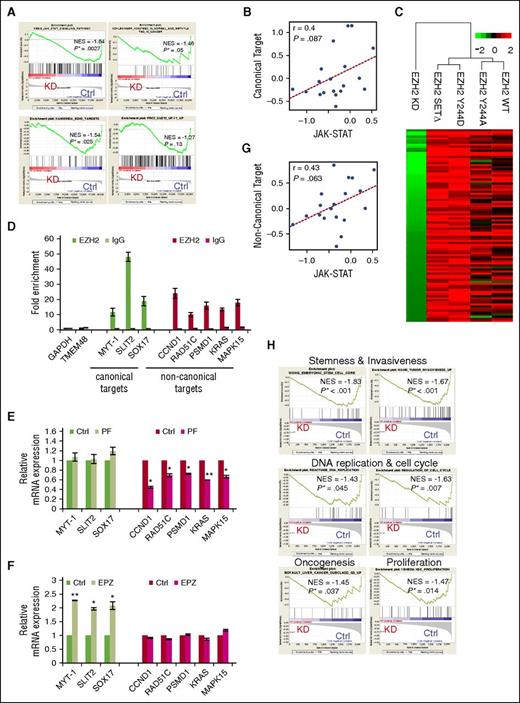
Phosphorylation of EZH2 by JAK3 is inhibitory for PRC2-mediated gene repression but required for gene activation toward proliferation. (A) GSEA plots show gene sets enriched among downregulated genes upon JAK3 knockdown. The 4 gene sets are described in Molecular Signatures Database: JAK-STAT signaling pathway; Genes bearing the H3K27me3 mark; Putative targets of EZH2 as an epigenetic silencer; Genes upregulated upon knockdown of SUZ12. (B) Correlation plot between the JAK-STAT gene signature and PRC2 canonical target gene signature indices in a dataset of NKTL patient samples (GSE80632). Each dot is a patient sample. Correlation coefficient and correlation test P value are also shown. (C) Hierarchical clustering of gene expression profiles of EZH2 KD, EZH2 WT, and it mutants (Y244A, Y244D, and SET∆) based on global profiling (GSE75680). Heat map shows expression profiles of noncanonical targets cross EZH2 KD, EZH2 WT, and its mutants (Y244A, Y244D, and SET∆) compared with their corresponding controls. (D) ChIP-qPCR assay in NKYS cells for endogenous EZH2 binding. RT-PCR was performed with immunoprecipitated chromatin fragments obtained by using an anti-EZH2 antibody or an irrelevant antibody (immunoglobulin G, IgG) as a control. (E) qRT-PCR analysis showing that JAK3 inhibitor PF decreased expression of noncanonical targets of EZH2. n = 4 independent experiments, means ± SD. *P < .05; **P < .01 (Student t test). (F) qRT-PCR analysis showing that gene expression of EZH2 noncanonical targets was not affected by PRC2 inhibitor EPZ-6438 (EPZ). The RNA harvested from NKYS cells exposed to EPZ at 200 nM was isolated, reverse-transcribed, subjected to qPCR by using primers as indicated, and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). n = 5 independent experiments. Results are means ± SD. *P < .05; **P < .01 (Student t test). (G) Correlation plot between the JAK-STAT gene signature and EZH2 noncanonical target gene signature indices in a dataset of NKTL patient samples (GSE80632). Each dot is a patient sample, and no cell line is presented. Correlation coefficient and correlation test P value are also shown. (H) GSEA plots of significantly enriched gene sets show the regulation of oncogenesis-related genes and pathways by EZH2 noncanonical function. KD, EZH2 knockdown; NES, normalized enrichment score.
Phosphorylation of EZH2 by JAK3 is inhibitory for PRC2-mediated gene repression but required for gene activation toward proliferation. (A) GSEA plots show gene sets enriched among downregulated genes upon JAK3 knockdown. The 4 gene sets are described in Molecular Signatures Database: JAK-STAT signaling pathway; Genes bearing the H3K27me3 mark; Putative targets of EZH2 as an epigenetic silencer; Genes upregulated upon knockdown of SUZ12. (B) Correlation plot between the JAK-STAT gene signature and PRC2 canonical target gene signature indices in a dataset of NKTL patient samples (GSE80632). Each dot is a patient sample. Correlation coefficient and correlation test P value are also shown. (C) Hierarchical clustering of gene expression profiles of EZH2 KD, EZH2 WT, and it mutants (Y244A, Y244D, and SET∆) based on global profiling (GSE75680). Heat map shows expression profiles of noncanonical targets cross EZH2 KD, EZH2 WT, and its mutants (Y244A, Y244D, and SET∆) compared with their corresponding controls. (D) ChIP-qPCR assay in NKYS cells for endogenous EZH2 binding. RT-PCR was performed with immunoprecipitated chromatin fragments obtained by using an anti-EZH2 antibody or an irrelevant antibody (immunoglobulin G, IgG) as a control. (E) qRT-PCR analysis showing that JAK3 inhibitor PF decreased expression of noncanonical targets of EZH2. n = 4 independent experiments, means ± SD. *P < .05; **P < .01 (Student t test). (F) qRT-PCR analysis showing that gene expression of EZH2 noncanonical targets was not affected by PRC2 inhibitor EPZ-6438 (EPZ). The RNA harvested from NKYS cells exposed to EPZ at 200 nM was isolated, reverse-transcribed, subjected to qPCR by using primers as indicated, and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). n = 5 independent experiments. Results are means ± SD. *P < .05; **P < .01 (Student t test). (G) Correlation plot between the JAK-STAT gene signature and EZH2 noncanonical target gene signature indices in a dataset of NKTL patient samples (GSE80632). Each dot is a patient sample, and no cell line is presented. Correlation coefficient and correlation test P value are also shown. (H) GSEA plots of significantly enriched gene sets show the regulation of oncogenesis-related genes and pathways by EZH2 noncanonical function. KD, EZH2 knockdown; NES, normalized enrichment score.
To investigate whether phosphorylation of EZH2 is required for the switch to a noncanonical function that activates gene expression, we assessed the gene expression of NKYS cells ectopically expressing EZH2 WT or its mutants, including the SET domain deletion mutant (EZH2 SET∆), which lacked the methyltransferase activity for H3K27me3 while keeping its noncanonical transcriptional activation function intact,6 EZH2 Y244A mutant (phospho-deficient) and Y244D mutant (phospho-mimic). Interestingly, the EZH2 Y244D mutant clusters with EZH2 SET∆, and they demonstrate a relative increase in general gene expression compared with EZH2 WT and the phosphorylation dead mutant Y244A (Figure 3C), supporting the hypothesis that the Y244 phosphorylation of EZH2 is associated with active transcriptional activity independent of histone methyltransferase activity. To characterize potential noncanonical EZH2 targets, we identified active genes that are downregulated by EZH2 siRNA, but upregulated upon expression of Y244D mutant and EZH2 SET∆. Ninety-three genes fulfilled these criteria (supplemental Table 1). To validate these EZH2-associated noncanonical targets, we performed chromatin immunoprecipitation-quantitative polymerase chain reaction (ChIP-qPCR) analysis of selected genes from the list. As expected, EZH2 occupancy in the promoters of previously identified PRC2-target genes MYT-1, SOX-17,5 and SLIT25 was detected. Importantly, a few previously unknown targets selected from the noncanonical target list, PMSD1, KRAS, and MAPK15 genes, also show significant enrichments of EZH2 (Figure 3D; supplemental Figure 3A). JAK3 inhibitor treatment led to a decrease in mRNA expressions of noncanonical target genes CCND1, RAD51C,5 PMSD1, KRAS, and MAPK15, although the canonical PRC2-target genes remained unchanged (Figure 3E; supplemental Figure 3B). Conversely, EZH2 enzymatic inhibitor EPZ-643815 reactivated transcription of the canonical PRC2-target genes but did not affect these 5 EZH2 noncanonical targets (Figure 3F; supplemental Figure 3C). Moreover, EZH2 noncanonical target gene signature constructed using these 5 validated noncanonical genes shows positive correlation with the JAK-STAT target signature in the dataset of patient samples (Figure 3G). These results further confirm the gene activation by JAK3- phosphorylated EZH2.
GSEA revealed a number of oncogenic pathways, such as “proliferation and biosysthesis,” “stemness and invasiveness,” “oncogenesis,” and “DNA replication and cell cycle,” overrepresented by these EZH2-noncanonical genes (Figure 3H; supplemental Table 2). These activated genes may therefore contribute to the proliferative phenotype observed in NKTL and 293T that requires EZH2 Y244 phosphorylation (Figure 2D; supplemental Figure 2F). Taken together, we propose that JAK3-mediated phosphorylation of EZH2 at Y244 suppresses its canonical epigenetic silencing function but promotes its noncanonical transcriptional activation function on a different set of genes that are implicated in cancer, which contributes to the oncogenic property of EZH2 in NKTL.
JAK3-mediated phosphorylation of EZH2 promotes the formation of EZH2-Pol II complex and interferes with its binding to SUZ12 and EED
Next, we explored the mechanism of the switch in EZH2 function from a polycomb group repressor to a transcriptional coactivator upon phosphorylation by JAK3. SUZ12, a core component of PRC2, failed to pull down JAK3, suggesting that JAK3-EZH2 complex could be distinct from the conventional PRC2 complex (supplemental Figure 4A). We therefore tested if EZH2 exists in a transcription activating complex. Notably, we observed that EZH2 IP can pull down Pol II (Figure 4A; supplemental Figure 4B). Due to the association of JAK3 with EZH2, JAK3 may also be associated with the Pol II-EZH2-containing complex. Using antibodies specific to endogenous JAK3 and immunoblotting for Pol II, we showed that Pol II strongly coprecipitated with JAK3. This interaction is confirmed by reciprocal Pol II IP experiments followed by immunoblotting for JAK3 (Figure 4B; supplemental Figure 4C). The physical associations among JAK3, EZH2, and Pol II support the idea that phosphorylated EZH2 directly participates in transcriptional gene regulation by interacting with transcriptional machinery. The interaction between EZH2 and Pol II was less detected in Y244A than in WT, suggesting that the phosphodeficiency impedes EZH2’s access to Pol II, while it increased the EZH2 association with the PRC2 components SUZ12 and EED (Figure 4C). This result suggests that Y244 phosphorylation of EZH2 interferes with its binding to other PRC2 subunits and thus provides a molecular explanation for the global decrease in H3K27me3 levels associated with JAK3 activation. Consistent with this, we observed that the Y244A mutant had less binding on the promoters of EZH2-activated targets that we identified in Figure 3, such as CCND1, RAD51C, PMSD1, KRAS, and MAPK15, while EZH2 binding to the canonical PRC2-target genes, such as MYT-1, SOX-17, and SLIT2, was not significantly affected (Figure 4D). To estimate the impact of EZH2 Y244 modification on gene expression, we tested how these EZH2-bound genes are differentially regulated with the Y244A mutant using quantitative reverse transcription polymerase chain reaction (qRT-PCR) assays. Consistent with the downregulation by JAK3 inhibitor (Figure 3E), Y244A mutant reduced expression of all these EZH2-activated genes CCND1, PMSD1, KRAS, and MAKP15, while it did not change expression levels of PRC2-targets, MYT-1, SOX-17, and SLIT2 (Figure 4E). Collectively, these analyses support the conclusion that phosphorylation of Y244 favors the association of EZH2 with Pol II and binding to a set of genes distinct from canonical PRC2 targets, leading to transcription activation.
EZH2 Y244A increases the interactions of EZH2 with PRC2 subunits and reduces the interaction with Pol II. (A) Coimmunoprecipitation assay shows the interaction between endogenous EZH2 and Pol II in NKTL cell lines. (B) Coimmunoprecipitation assay shows the interaction between endogenous Pol II and JAK3 in NKYS cells. (C) Coimmunoprecipitation assay shows the changes in the association of EZH2 WT /Y244A with Pol II, SUZ12, and EED. 293T cells were cotransfected with JAK3 A572V and EZH2 WT/Y244A expression plasmids or control vector for 48 hours. (D) ChIP-qPCR assay in 293T cells for binding of ectopically expressed EZH2WT/EZH2 Y244A to EZH2 target genes. ChIP assays were performed by using 293T cells cotransfected by JAK3 A572V and EZH2 WT/Y244A expression plasmids. Real-time PCR was performed with immunoprecipitated chromatin fragments obtained by using an anti-HA antibody. Results are means ± SD. n = 3 independent experiments. *P < .05; **P < .01 (Student t test). (E) qRT-PCR assay in 293T cells showing that EZH2-Y244A mutation affects EZH2 target gene expression. Results are means ± SD. n = 5 independent experiments. *P < .05; **P < .01 (Student t test).
EZH2 Y244A increases the interactions of EZH2 with PRC2 subunits and reduces the interaction with Pol II. (A) Coimmunoprecipitation assay shows the interaction between endogenous EZH2 and Pol II in NKTL cell lines. (B) Coimmunoprecipitation assay shows the interaction between endogenous Pol II and JAK3 in NKYS cells. (C) Coimmunoprecipitation assay shows the changes in the association of EZH2 WT /Y244A with Pol II, SUZ12, and EED. 293T cells were cotransfected with JAK3 A572V and EZH2 WT/Y244A expression plasmids or control vector for 48 hours. (D) ChIP-qPCR assay in 293T cells for binding of ectopically expressed EZH2WT/EZH2 Y244A to EZH2 target genes. ChIP assays were performed by using 293T cells cotransfected by JAK3 A572V and EZH2 WT/Y244A expression plasmids. Real-time PCR was performed with immunoprecipitated chromatin fragments obtained by using an anti-HA antibody. Results are means ± SD. n = 3 independent experiments. *P < .05; **P < .01 (Student t test). (E) qRT-PCR assay in 293T cells showing that EZH2-Y244A mutation affects EZH2 target gene expression. Results are means ± SD. n = 5 independent experiments. *P < .05; **P < .01 (Student t test).
JAK3 inhibitor is robust in inhibiting NKTL while EZH2 inhibitors are not efficacious
EZH2 is emerging as a therapeutic target in cancer. A number of EZH2 catalytic inhibitors or inhibitors that disrupt EZH2-EED protein-protein interactions have been developed, and some are already in clinical trials. In this study, we investigated GSK126,16 EPZ-6438,15 and SAH-EZH22 using cell viability assay and found that either catalytic inhibition of EZH2 or disruption of PRC2 complex was insufficient to affect the viability of NKTL cells (Figure 5B-C,E-F; supplemental Figure 5A,C), although all 3 inhibitors effectively downregulated H3K27me3 (Figure 5A,D,G; supplemental Figure 5B). These results confirm our previous finding that EZH2’s oncogenic function in NKTL is independent of its histone methylating activity.6
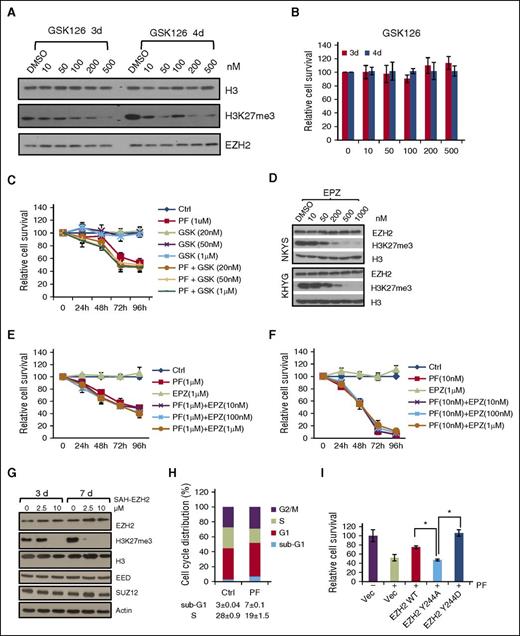
JAK3 Inhibitor is robust in inhibiting NKTL while EZH2 inhibitors are not efficacious. (A) Immunoblot analysis of NKYS cells treated with EZH2 inhibitor GSK126 (GSK) at indicated concentration for 3 days and 4 days. (B) Cell viability of NKYS cells treated with GSK. (C) Cell viability of NKYS cells treated with PF and GSK combination. No obvious synergistic effect. (D) Immunoblot analysis of NKTL cells treated with EZH2 inhibitor EPZ. (E-F) Cell viability assay of NKTL cells treated with EPZ as a single agent or combination with PF. (E) for NKYS and (F) for KHYG. (G) Immunoblot analysis of KHYG cells treated with EZH2 inhibitor SAH-EZH2. Cells were treated with SAH-EZH2 twice daily. (H) Cell cycle analysis of NKYS cells treated with PF for 24 hours. (I) Cell viability assay of PF-treated NKYS cells ectopically expressing EZH2 WT/Y244A/Y244D. Data are presented as mean values ± SD of triplicates. *P < .05 (Student t test). DMSO, dimethyl sulfoxide.
JAK3 Inhibitor is robust in inhibiting NKTL while EZH2 inhibitors are not efficacious. (A) Immunoblot analysis of NKYS cells treated with EZH2 inhibitor GSK126 (GSK) at indicated concentration for 3 days and 4 days. (B) Cell viability of NKYS cells treated with GSK. (C) Cell viability of NKYS cells treated with PF and GSK combination. No obvious synergistic effect. (D) Immunoblot analysis of NKTL cells treated with EZH2 inhibitor EPZ. (E-F) Cell viability assay of NKTL cells treated with EPZ as a single agent or combination with PF. (E) for NKYS and (F) for KHYG. (G) Immunoblot analysis of KHYG cells treated with EZH2 inhibitor SAH-EZH2. Cells were treated with SAH-EZH2 twice daily. (H) Cell cycle analysis of NKYS cells treated with PF for 24 hours. (I) Cell viability assay of PF-treated NKYS cells ectopically expressing EZH2 WT/Y244A/Y244D. Data are presented as mean values ± SD of triplicates. *P < .05 (Student t test). DMSO, dimethyl sulfoxide.
Because JAK3-directed noncanonical function of EZH2 seems to be predominant in NKTL, we hypothesize that JAK3 inhibition would be an effective therapy in NKTL. Indeed, treatment of NKYS cells with JAK3 kinase inhibitor PF956980 dramatically decreased cell growth as demonstrated by cell viability assay (Figure 5C,E-F). At the same time, there was a reduction in cells undergoing S phase and more cells undergo cell death (Figure 5H). Of note, the combination of PRC2 catalytic inhibitors with JAK3 inhibitor had very little additional effects compared with JAK3 inhibitor alone (Figure 5C,E-F). Importantly, the growth inhibitory effect of JAK3 inhibitor was fully rescued by phosphomimic EZH2 mutant (Y244D), but not by phosphodeficient Y244A mutant (Figure 5I). This result suggests that the observed growth-inhibitory effect of JAK3 inhibition is mediated through suppression of phosphorylation-associated noncanonical EZH2 function. Consistently, comparison of enriched gene sets or pathways in JAK3 and EZH2 WT versus their knockdown reveals that they share 92 significantly enriched gene sets, which are under 7 gene ontology categories of biological processes or pathways, including “Enhanced stemness and invasiveness,” “Oncogenesis,” “Enhanced DNA replication and cell cycle,” “Enhanced proliferation and biosynthesis,” “Enhanced RNA process,” and “Drug responses” (supplemental Table 3). Thus, from a translational point of view, these results suggest a potential application of inhibiting JAK3 to treat NKTL patients with high levels of EZH2, which might be worthy of clinical exploration.
Discussion
Dissecting the molecular pathways that drive noncanonical function of EZH2 in NKTL is important to improve our understanding of the biology of this aggressive cancer and to develop better therapeutic strategies. In this study, we show that EZH2 is tyrosine-phosphorylated by JAK3 at Y244, and this modification causes it to dissociate from PRC2 complex, leading to EZH2 oncogenic function independent of its enzymatic activity. Our findings therefore suggest a novel model of JAK3-mediated oncogenic effect via posttranslational modification of EZH2 at a specific residue, thereby changing its function from a gene repressor to a gene activator of distinct genes involved in malignant phenotype. JAK3 inhibitors, which are currently under clinical development, inhibit NKTL cell growth through prevention of EZH2 phosphorylation at the Y244 residue and may be a useful therapeutic intervention (Figure 6).
Model for EZH2 functional switch from a polycomb repressor to a transcriptional activator in NK lymphoma. JAK3 overactivation leads to phosphorylation of EZH2 at Y244. This phosphorylation event shifts EZH2 from a transcriptional repressor associated with PRC2 to a transcriptional coactivator cooperating with Pol II. This study suggests that blocking EZH2 phosphorylation by JAK3 inhibitor (JAK3i) is a potential therapeutic strategy to inhibit EZH2 noncanonical oncogenic function in NKTL. PcG, polycomb group.
Model for EZH2 functional switch from a polycomb repressor to a transcriptional activator in NK lymphoma. JAK3 overactivation leads to phosphorylation of EZH2 at Y244. This phosphorylation event shifts EZH2 from a transcriptional repressor associated with PRC2 to a transcriptional coactivator cooperating with Pol II. This study suggests that blocking EZH2 phosphorylation by JAK3 inhibitor (JAK3i) is a potential therapeutic strategy to inhibit EZH2 noncanonical oncogenic function in NKTL. PcG, polycomb group.
The established oncogenic function of EZH2 is primarily through silencing of tumor-suppressor genes by H3K27 trimethylation. However, there has been emerging evidence that EZH2 may also function as a transcriptional activator through different molecular mechanisms in different cancer models. In breast cancer cells, EZH2 has been reported to act as a transcriptional activator but its mechanism of action depends on the cell type. In estrogen receptor-positive breast cancer cells, EZH2 interacts directly with estrogen receptor and β-catenin, functionally enhancing gene transactivation in the estrogen and Wnt pathways.3 In triple-negative breast cancer cells, EZH2 can activate transcription of the NF-kB target genes by forming a ternary complex with RelA and RelB.4 In colon cancer, the proliferating cell nuclear antigen-associated factor PAF binds to β-catenin and recruits EZH2 at Wnt target genes to induce their expression.17 In NKTL, our previous report6 and the current study demonstrate that EZH2 activates genes, which contribute to higher proliferative capacity of NK cells. However, in contrast to what was described in these studies, in which EZH2’s transcriptional activation activity is independent of the EZH2 SET domain and H3K27me3, the transcriptional properties of EZH2 in castration-resistant prostate cancer rely on its methyltransferase activity, but not the other PRC2 components.5 In summary, these findings, including ours, suggest an important role of EZH2 as a transcription activator in a wide range of malignancies, yet its exact mechanisms and deregulated pathways seem to be cell context-dependent. Importantly, the molecular switch to gene activation function is less clearly defined.
Previous studies have noticed that H3K27me3 expression is significantly lower in breast, ovarian, and pancreatic cancers than in normal tissues.18 Although the molecular mechanisms underlying the loss of H3K27me3 in these tumors remain elusive, increasing evidence suggests that the EZH2 protein modification may account for this.19,20 NKTL is a malignancy driven by constitutive activation of JAK3. In our study, we found that modification of EZH2 by JAK3 results in the disruption of the integrity of the PRC2 complex (and EZH2 forming new complex with other proteins), resulting in a reduction in the methyltransferase activity and specificity toward H3K27. We found a significant correlation between JAK/STAT activation and increase expression of canonical PRC2 genes in our Asian NKTL patients. Our results are consistent with reports showing that most NKTL expressed phosphorylated JAK3 regardless of their JAK3 mutation status,9 and several gene sets related to the PRC2 complex were enriched among these upregulated genes.21 Conversely, phosphorylated EZH2 associates with Pol II at the promoters of noncanonical target genes and “actively” regulate the expression of these genes. We further elucidated that the phosphorylation of Y244 residue on EZH2 is the key molecular mediator of this switch between the PRC2-mediated canonical function of repressing polycomb group-target genes and the noncanonical function of transcriptional activation of distinct genes mediated via EZH2’s association with transcriptional complex.
Phosphorylation is considered to be one of the key steps in signal transduction and regulation of enzymatic activity, and growing evidence shows that EZH2 activity and stability are tightly regulated by this posttranslational modification. AKT was shown to phosphorylate EZH2 at serine 21 and inhibit its enzyme activity for H3K27me3.19 This phosphorylation site was shown to be critical for the H3K27me3-independent function of EZH2 in castration-resistant prostate cancer5 and glioblastoma.22 Several independent studies have identified EZH2 residues phosphorylated by CDK1/2 at multiple sites, including T345, T416, and T487 with diverse regulations, and may depend on cell types and conditions.20,23-25 Intriguingly, JAK2 has been found in a recent study to phosphorylate EZH2 at tyrosine 641 (Y641), which promotes the interaction of EZH2 with E3 ubiquitin ligase β-TrCP and degradation of EZH2.26 On the other hand, our study in NKTL shows a distinct mechanism by which JAK3-mediated EZH2 phosphorylation at Y244 converts EZH2 silencing activity into an activating effector. We point out that the possibility of Y641 as a potential phosphorylation site in EZH2 mediating the effects of JAK3 in NKTL can be ruled out for 3 reasons. First, the reported JAK2-mediated EZH2 Y641 phosphorylation results in proteasomal degradation of EZH2, whereas EZH2 protein level is not affected by JAK3 in NKTL (Figure 1C-F). Second, Y641 is in the SET domain of EZH2 protein; however, even the deletion of SET domain cannot impair the ability of EZH2 protein to confer cell growth advantage in NKTL.6 Last, EZH2 Y244A mutant is sufficient to abolish the tyrosine-phosphorylation of EZH2 mediated by JAK3 activation (Figure 2F). In summary, posttranslational modifications of EZH2 therefore regulate its function in cells and may be the critical mechanism modulating its oncogenic function in different cancers.
Recent discoveries of recurrent somatic EZH2 mutations in myelodysplastic syndromes and myeloproliferative neoplasms indicate that inactivation of EZH2 may contribute to the pathogenesis of myeloid malignancies, which would presumably be associated with loss of H3K27 trimethylation at specific gene targets. It is noteworthy that a striking correlation exists between low H3K27me3 expression and poor prognosis in patients with breast, ovarian, and pancreatic cancer.18 In gliomas, aberrant epigenetic silencing through H3K27me3-mediated inhibition of PRC2 activity promotes oncogenesis.27,28 In addition, direct EZH2 inhibition by small molecules targeting the enzyme active site may have detrimental effects on global methylation patterns in normal cells. All these could lead to concerns about the use of EZH2 enzymatic inhibitors. Furthermore, considering a tumor-suppressor function for EZH2 in some cellular contexts,29-31 indiscriminate depletion of EZH2 protein or inhibition of EZH2 function may be problematic. Understanding the complex mechanisms that regulate EZH2 activity in tumor cells may help design better therapeutic intervention strategies. Our study suggests that inhibition of JAK3-mediated phosphorylation of EZH2 may be an alternative therapeutic option to diminish EZH2 oncogenic activity in cancer cells without reducing the global EZH2-mediated gene silencing.
JAKs are critical components of many cytokine receptor systems: regulating growth, survival, differentiation, and immune response. Researchers have found that dysregulated cytokine signaling contributes to cancer. Our study reveals a mechanistic link between JAK3 and EZH2-mediated oncogenesis, 2 important oncogenic pathways in NKTL. The data provide evidence that JAK3 directly phosphorylates EZH2 at a novel site, and this modification confers EZH2 noncanonical function. Deregulation of the epigenetic machinery and gene regulation may therefore represent an additional key mechanism by which JAK3 contributes to cancer. Of importance is that JAK3 is aberrant in several hematologic malignancies and solid tumors, such as colon32,33 and breast carcinoma,33-35 and seems to be of functional relevance in these cancers. Because tumors in which JAK3 is activated overlaps with those with reduced H3K27me3 and EZH2 overexpression, the findings in our study could have broader utility and implications outside NKTL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kara Johnson at Oregon Health and Science University–Knight Cancer Institute for the MIG-JAK3 (wild-type) and MIG-JAK3 (A572V) plasmids and Lim Soon Thye and Ong Choon Kiat from the National Cancer Center Singapore for the NK-S1 cell line. The authors thank Zit Liang Chan for technical assistance in gene expression microarray and Daniel G. Tenen, H. Phillip Koeffler, Sudhakar Jha, and Motomi Osato for critical comments.
This study was supported by the National Medical Research Council (NMRC) grants NMRC/Clinician Scientist-Individual Research/1343/2012 (W.-J.C.) and NMRC/Basic Research Grant-New Investigator/2021/2014 (J.Y.). W.-J.C. was also supported by the National Medical Research Council Clinician Scientist Investigator Award.
Authorship
Contribution: J.Y. and W.-J.C. conceived, designed, and supervised the study. B. Li, B. Lin, P.T.L., J.T., C.B., V.S., and X.T.L. performed experiments. J.Y., B. Li, T.-H.C., and H.Y. analyzed and interpreted data. S.-B.N. provided gene expression data on clinical samples. J.Y., Q.Y., and W.-J.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wee-Joo Chng, National University of Singapore, 5 Lower Kent Ridge Rd, Singapore 119074; e-mail: mdccwj@nus.edu.sg.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal