Key Points
Survival of acute myelogenous leukemia is frequently limited by pneumonia, due to disease- and therapy-associated immune defects.
Inducible epithelial resistance protects neutropenic, leukemic mice against lethal pneumonia without impacting AML cell proliferation.
Abstract
Despite widespread infection prevention efforts, pneumonia remains the leading cause of death among patients with acute leukemia, due to complex disease- and treatment-dependent immune defects. We have reported that a single inhaled treatment with a synergistic combination of Toll-like receptor 2/6 (TLR 2/6) and TLR9 agonists (Pam2-ODN) induces protective mucosal defenses in mice against a broad range of pathogens. As Pam2-ODN–induced protection persists despite depletion of several leukocyte populations, we tested whether it could prevent pneumonia in a mouse model of acute myeloid leukemia (AML) remission induction therapy. Pam2-ODN prevented death due to pneumonia caused by Pseudomonas aeruginosa, Streptococcus pneumoniae, and Aspergillus fumigatus when mice were heavily engrafted with leukemia cells, had severe chemotherapy-induced neutropenia or both. Pam2-ODN also extended survival of pneumonia in NSG mice engrafted with primary human AML cells. Protection was associated with rapid pathogen killing in the lungs at the time of infection and with reduced pathogen burdens at distant sites at the end of observation. Pathogen killing was inducible directly from isolated lung epithelial cells and was not abrogated by the presence of leukemia cells or cytotoxic agents. Pam2-ODN had no discernible effect on replication rate, total tumor population, or killing by chemotherapy of mouse or human leukemia cells, either in vitro or in vivo. Taken together, we report that therapeutic stimulation of lung epithelial defenses robustly protects against otherwise lethal pneumonias despite the profound immune dysfunction associated with acute leukemia and its treatment. These findings may suggest an opportunity to protect this population during periods of peak vulnerability.
Introduction
Among both healthy and immunosuppressed people worldwide, pneumonia is a leading cause of premature death and disability,1-4 and nosocomial pneumonias cause more deaths than any other hospital infection.5 Patients with acute myelogenous leukemia (AML) or high-risk myelodysplastic syndrome (MDS) face a particular pneumonia risk, as both disease and treatment impair immune function.6-11 In the transfusion era, autopsy studies reveal that pneumonia is the most frequent cause of death among leukemia patients,12,13 and recent studies find that the presence of pneumonia is the leading hazard for death during leukemia remission induction therapy.14 Moreover, these figures fail to capture leukemia-related deaths caused by withholding essential myeloablative therapies due to concerns about immunosuppression in patients with suspected lung infections. Further, current unacceptably high rates of pneumonia persist despite widespread use of environmental hygiene protocols and prophylactic antibiotics. Thus, although enhanced control of pneumonia would substantially enhance the chance of long-term survival for patients with acute leukemias, novel strategies are necessary to achieve success in this regard.
One theoretically appealing approach to improve pneumonia-related outcomes in patients with leukemia is to preferentially augment those host defense elements that are relatively less impaired by the disease process. Leukemia patients frequently present with complex leukocyte defects, often arising from both multilineage cytopenias and functional impairments of chemotaxis, diapedesis, and pathogen killing.15-17 Thus, therapeutic manipulation of lung parenchymal cells might present an opportunity to protect patients against pneumonia without reliance on the cells most negatively impacted by disease and treatment.
To this end, we reported that therapeutic ligation of pattern recognition receptors in the lungs can stimulate protective antimicrobial responses directly from lung epithelial cells, a phenomenon termed inducible resistance.18-22 Given the relative tolerance of lung epithelial cells to immunosuppressive therapies23,24 and our observation that inducible resistance persists despite leukocyte depletion,25-27 we hypothesized that this strategy could protect against pneumonia in the setting of leukemia and leukemia therapy. Here, we present evidence that inducible resistance persists in vitro and in vivo, despite exposure to leukemia and common antileukemia chemotherapy and propose this novel approach as a means to improve survival of patients with AML/MDS.
Methods
Animals and reagents
All mice were handled in accordance with the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. Experiments were done in 5- to 10-week-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Mice with LoxP sites flanking exon 3 of Myd88 were kindly provided by Anthony L. DeFranco28 and Sftpc-Cre mice were kindly provided by Brigid L. M. Hogan.29 For primary human AML xenograft models, 8- to 12-week-old NOD SCID γ (NSG; NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice were used (The Jackson Laboratory). All general reagents were purchased from Sigma-Aldrich (St Louis, MO) unless stated otherwise.
In vivo Pam2-ODN treatments
As previously reported,18 a combination of 4 µM S-[2,3-bis(palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl) 3-lysine (Pam2CSK4) and 1 µM of ODN M362 (InvivoGen, San Diego, CA), hereafter referred to as Pam2-ODN, were diluted in 10 mL phosphate-buffered saline (PBS), placed in an Aerotech II nebulizer (Biodex, Shirley, NY), and delivered to unrestrained mice in an exposure chamber via polyethylene tubing. Nebulization was driven by 10 L/min air supplemented with 5% CO2 for 20 minutes.
In vivo infection models
As previously described,25 frozen stock of Pseudomonas aeruginosa strain PA103 (American Type Culture Collection, Manassas, VA) was incubated overnight in tryptic soy broth and then expanded in Luria-Bertini media to OD600 0.35. Mouse-adapted Streptococcus pneumoniae serotype 4 isolated from a patient with pneumonia, kindly provided by Daniel A. Musher, was incubated overnight in Todd-Hewett broth (Becton-Dickinson) with defribinated sheep red blood cells (Becton-Dickinson) and then expanded in brain heart infusion to OD600 0.75. Bacterial suspensions were centrifuged, washed, resuspended in PBS, and aerosolized over 60 minutes. For all bacterial challenges, a nebulized inoculum of 10 mL of ∼2 × 1010 colony-forming units (CFU)/mL were delivered, except in challenges delivered to NSG mice, where the inoculum was reduced to 10 mL of ∼9 × 109 CFU/mL P. aeruginosa. Immediately after bacterial challenges, some mice were anesthetized, and their lungs were harvested and homogenized25 using a Mini-Beadbeater-1 (Biospec, Bartlesville, OK). Serial dilutions of the nebulizer inoculum and lung homogenates were plated on tryptic soy agar plates (Becton Dickinson). The remaining mice were observed for survival/euthanasia criteria for 12 days.
As described,19 Aspergillus fumigatus strain AF293 (American Type Culture Collection) was plated on yeast extract agar for 3 days and then collected by gentle agitation in 0.1% Tween-20 PBS. The suspension was filtered, centrifuged, washed, and resuspended in 10 mL PBS; 109 conidia/mL were nebulized for 60 minutes. Lungs were harvested 24 hours after infection. The right lung was fixed in 10% paraformaldehyde, paraffin embedded, cut, and stained with Gomori Modified Silver (GMS). For GMS stain quantification, color threshold was established by positive silver stain area selection, and settings for hue, saturation, and brightness were standardized between groups in ImageJ (National Institutes of Health, Bethesda, MD). The stained area of four 40× fields per condition was measured, and ratios of GMS positive:background stain were calculated. The left lung was homogenized as described above and centrifuged, and the supernatant was plated in a Platelia Galactomannan ELISA plate (Bio-Rad Laboratories, Hercules, CA), which was read according to the manufacturer’s protocol.
In vitro infectious challenges
Approximately 3 × 104 MLE15 mouse lung epithelial cells, kindly provided by Jeffrey A. Whitsett, were plated on the apical side of 0.33-cm2 transwell plates (Corning-Life Sciences, Corning, NY). Once confluence was reached, cells were treated with 9.3 µM Pam2CSK4 and 2.2 µM ODNM362 or PBS. Approximately 8 × 104 FBL3 cells were seeded in the basolateral chamber of the culture well. FBL3 cells were later treated with cytarabine arabinoside (Ara; 625 µg/mL) and idarubicin (Ida; 50 µg/mL) or media alone. After treatment, cells were infected with A. fumigatus (0.1 multiplicity of infection, 3 × 103 conidia) or P. aeruginosa (20 μL × 1 × 105 CFU/mL) and incubated for 4 hours.
Mouse tracheal epithelial cell harvest
For tracheal epithelial cell harvests, mice were anesthetized, and tracheas were exposed, excised, digested as previously described,30 and plated on collagen-coated transwell inserts. After 7 days, apical media were aspirated, and basal media were changed to differentiation media to achieve air–liquid interface. After an additional 7-day differentiation, cells were treated and infected as described above.
Quantitative RT-PCR analysis and primers
HBEC3kt cells, kindly provided by John D. Minna, were treated with Pam2-ODN or DMEM with 1% penicillin-streptomycin (base media). After 20 minutes, treated media were replaced with base media. RNA was extracted at the indicated time points using RNeasy Mini Kit (Qiagen, Valencia, CA); 500 ng total RNA from the samples was reversed transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad). cDNA was quantified by reverse transcriptase (RT)-polymerase chain reaction (PCR) using SYBR green PCR master mix (Applied Biosystems, Life Technologies) and measured on an ABI ViiA 7 Real-Time PCR System. Genes were normalized to 18s transcript levels. Primers for 18S (5′-GTAACCCGTTGAACCCCATT-3′) (5′-CCATCCAATCGGTAGTAGCG-3′), LCN2 (5′-GTGAGCACCAACTACAACCAGC-3′) (5′-GTTCCGAAGTCAGCTCCTTGGT-3′), CXCL2 (5′-GGCAGAAAGCTTGTCTCAACCC-3′) (5′-CTCCTTCAGGAACAGCCACCAA-3′), and S100A8 (5′-ATGCCGTCTACAGGGATGACCT-3′)(5′-AGAATGAGGAACTCCTGGAAGTTA-3′) were purchased from Sigma-Aldrich.
In vivo chemotherapy treatments
Initial Ara and Ida doses for mice were converted from human dosing (Ara 100–200 mg/m2, Ida 12 mg/m2) using Dose (mg/m2) = Km (dose in mg/kg) provided by the US Food and Drug Administration Guidance for Industry,31 where Km (converting factor) is 3 for mice. This calculates to Ara 33 to 50 mg/kg and Ida 1 to 2 mg/kg. Mice were injected with separate intraperitoneal injections for each drug diluted in sterile PBS or PBS only (control). Based on titration to maximum tolerability and induction of severe neutropenia (<500 neutrophils/µL), all experiments were performed following 3 days of Ara 50 mg/kg and Ida 1 mg/kg. Tail vein leukocyte and neutrophil counts were recorded at baseline and day 4. Leukocyte counts were assessed by Coulter method, and a manual differential count was used to assess neutrophil levels.
FBL3 cell growth, viability, and proliferation
FBL3 cells, a Friend MuLV-induced green fluorescent protein (GFP)-expressing erythroleukemia line of C57BL/6 origin,32 kindly provided by Shulin Li, were grown in RPMI with 10% fetal bovine serum and 1% penicillin-streptomycin. FBL3 cells were incubated in 96-well plates and incubated with escalating doses of Pam2-ODN, Ara, Ida, Ara + Ida, or PBS. Cell survival was measured at 24, 48, and 72 hours after treatment with an XTT cell viability kit (Cell Signaling) or Trypan blue exclusion assay. Cell proliferation was measured by a 5-bromo-2′-deoxyuridine (BrdU) immunofluorescence kit (Hoffman-La Roche, Basel, Switzerland).
FBL3 in vivo leukemia model
FBL3 cells (1 × 106) were injected per mouse via tail vein. Weekly 100-μL retroorbital blood samples were lysed using ACK buffer (Lonza), centrifuged, resuspended in PBS, and submitted to flow cytometric analysis for GFP-positive cells until engraftment was confirmed. Once engraftment was confirmed, baseline total leukocyte and differential counts were recorded, and 3-day chemotherapy with Ara and Ida was delivered (or not). On day 4 of chemotherapy, leukocytes and neutrophils were again measured, and Pam2-ODN or PBS was nebulized for 20 minutes. On day 5, mice were submitted to infectious challenges. Some mice were immediately euthanized to assess lung pathogen burdens as above. The remaining mice were euthanized when moribund, had excessive weight loss, or per local veterinary orders. Lungs, blood, and spleen were harvested at the time of death, homogenized, serially diluted, and plated in tryptic soy agar plates.
In vivo NSG primary leukemia xenograft
NSG mice were injected with 1 x×106 primary human AML cells passaged once through mice. Mice were bled weekly, and engraftment was confirmed by the presence of human CD45+ cells. Once all mice achieved circulating leukemia, they were submitted to nebulized treatment and challenge. Given their profound immune suppression, mice were checked 3 to 4 times daily, and those that fulfilled euthanasia criteria were euthanized. Lung, spleen, and blood were harvested for pathogen burden quantification.
Statistical analysis
Statistical analysis was performed using SigmaPlot 12.5 (Systat Software, Chicago, IL). A 2-sided Student t test was used to compare significance between 2 continuous variables. When >2 continuous variables were compared, a 1-way analysis of variance was executed with a Bonferroni post hoc test. Survival was plotted using Kaplan-Meier function, and a log-rank test was used to compare distribution among groups. A probability of acceptance of the null hypothesis of P ≤ .05 was considered significant.
Results
Lung epithelial cells are necessary and sufficient to generate protective antimicrobial responses
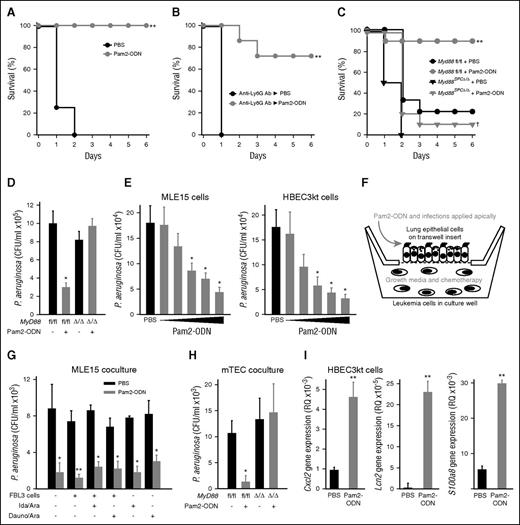
Mice treated by aerosol with a single dose of Pam2-ODN 24 hours before infectious challenge demonstrate robust protection against otherwise lethal infection with P. aeruginosa, if they are immunocompetent or if their circulating neutrophil levels have been depleted to undetectable levels with depleting anti-Ly6G antibody (Figure 1A-B).25 Conversely, mice conditionally deficient in Toll-like receptor (TLR) signaling in lung epithelial cells (Myd88SPC∆/∆) demonstrate complete abrogation of the Pam2-ODN–induced protection (Figure 1C), indicating a requirement for epithelial cells in inducible resistance. As in our prior work, the protection conferred by Pam2-ODN consistently correlates with rapid induction of pathogen killing in the lungs at the time of infection (Figure 1D), indicating that generation of an antimicrobial environment by the lung epithelium is important to the induced survival advantage. Dose-dependent Pam2-ODN–induced pathogen killing by isolated mouse (MLE15) and human (HBEC3kt) lung epithelial cells demonstrates the sufficiency of these cells to generate antimicrobial responses (Figure 1E). All subsequent in vitro experiments were performed with the median effective dose used in this experiment (Pam2 9.3 µM and ODN 2.16 µM).
Lung epithelial cells are necessary and sufficient to generate protective antimicrobial responses, even in the presence of leukemia cells and chemotherapy. (A) Wild-type C57BL6/J mice challenged with P. aeruginosa 24 hours after inhaled treatment with Pam2-ODN (4 µM Pam2 and 1µM ODN M362) or PBS (sham). (B) Neutrophil-depleted mice challenged with P. aeruginosa 24 hours after inhaled treatment with Pam2-ODN or PBS. (C) Lung epithelial MyD88 deletant mice challenged with P. aeruginosa 24 hours after inhaled treatment with Pam2-ODN or PBS. (D) Bacterial burden of lungs removed immediately after P. aeruginosa challenge in C. (E) Bacterial burden of MLE15 (top) and HBEC3kt (bottom) monolayer cultures 8 hours after infection with P. aeruginosa. Cell were exposed to the indicated treatment of 4 hours prior to infection. The escalating doses of Pam2 (µM):ODN (µM) were 0.31:0.072, 0.93:0.22; 3.1:0.72; 9.3:2.2; and 52:12. (F) Schematic of epithelial-leukemia coculture model. (G) Apical bacterial burden of MLE15 cultures grown in coculture with FBL3 cells (or not), in the presence or absence of the indicated chemotherapy. Cells were treated for 4 hours with Pam2-ODN (9.3 and 2.2 µM, respectively) or PBS, infected for 4 hours, and then samples were collected. (H) Apical bacterial burden of primary mouse tracheal epithelial cells from the indicated genotypes grown in coculture with FBL3 cells. Cells were treated for 4 hours with Pam2-ODN or PBS, infected for 4 hours, and then samples were collected. (I) Quantitative real-time PCR of HBEC3kt cells for inflammatory cytokine or antimicrobial peptides genes 30 minutes after treatment with Pam2-ODN or PBS. Shown are RQ values relative to 18s expression. All data are representative of ≥3 experiments. N = 8 to 10 mice for all groups in survival experiments, N = 4 mice or culture wells per group for bacterial burden experiments. *P < .05 vs PBS treated; **P < .005 vs PBS treated; †P < .005 vs Pam2-ODN–treated Myd88 fl/fl mice.
Lung epithelial cells are necessary and sufficient to generate protective antimicrobial responses, even in the presence of leukemia cells and chemotherapy. (A) Wild-type C57BL6/J mice challenged with P. aeruginosa 24 hours after inhaled treatment with Pam2-ODN (4 µM Pam2 and 1µM ODN M362) or PBS (sham). (B) Neutrophil-depleted mice challenged with P. aeruginosa 24 hours after inhaled treatment with Pam2-ODN or PBS. (C) Lung epithelial MyD88 deletant mice challenged with P. aeruginosa 24 hours after inhaled treatment with Pam2-ODN or PBS. (D) Bacterial burden of lungs removed immediately after P. aeruginosa challenge in C. (E) Bacterial burden of MLE15 (top) and HBEC3kt (bottom) monolayer cultures 8 hours after infection with P. aeruginosa. Cell were exposed to the indicated treatment of 4 hours prior to infection. The escalating doses of Pam2 (µM):ODN (µM) were 0.31:0.072, 0.93:0.22; 3.1:0.72; 9.3:2.2; and 52:12. (F) Schematic of epithelial-leukemia coculture model. (G) Apical bacterial burden of MLE15 cultures grown in coculture with FBL3 cells (or not), in the presence or absence of the indicated chemotherapy. Cells were treated for 4 hours with Pam2-ODN (9.3 and 2.2 µM, respectively) or PBS, infected for 4 hours, and then samples were collected. (H) Apical bacterial burden of primary mouse tracheal epithelial cells from the indicated genotypes grown in coculture with FBL3 cells. Cells were treated for 4 hours with Pam2-ODN or PBS, infected for 4 hours, and then samples were collected. (I) Quantitative real-time PCR of HBEC3kt cells for inflammatory cytokine or antimicrobial peptides genes 30 minutes after treatment with Pam2-ODN or PBS. Shown are RQ values relative to 18s expression. All data are representative of ≥3 experiments. N = 8 to 10 mice for all groups in survival experiments, N = 4 mice or culture wells per group for bacterial burden experiments. *P < .05 vs PBS treated; **P < .005 vs PBS treated; †P < .005 vs Pam2-ODN–treated Myd88 fl/fl mice.
Inducible epithelial antimicrobial responses persist despite exposure to leukemia cells and chemotherapy
To model in vitro the lung–leukemia interactions that occur in vivo, we developed a coculture model with polarized lung epithelial cells grown on semipermeable transwell inserts at air-liquid interface with FBL3 AML cells grown in the basal chamber (Figure 1F). Pam2-ODN (or sham) and infectious challenges were applied apically to model airway exposures, whereas leukemia cells, growth media, and chemotherapy treatments were administered to the basal chamber to model hematogenous exposures. Coculture with FBL3 cells did not impair the inducible epithelial bacterial killing, nor did exposure to chemotherapeutic agents commonly used to treat acute leukemias (idarubicin + cytarabine or daunorubicin + cytarabine; Figure 1G), although mouse tracheal epithelial cells harvested from TLR signaling–deficient mice demonstrated no ability to kill bacteria (Figure 1H). The Pam2-ODN–induced pathogen-killing response is characterized by expression of inflammatory chemokines and antimicrobial peptides beginning within 30 minutes of treatment, as shown for CXCL2, LCN2, and S100A8 in Figure 1I. Although we have reported MyD88-dependent induction of these and other host defense peptides,18,25,33 it appears likely that other classes of molecules (eg, reactive oxygen species18 ) and MyD88-independent signaling events also contribute to protection.
Pam2-ODN protects chemotherapy-treated mice against bacterial pneumonia.
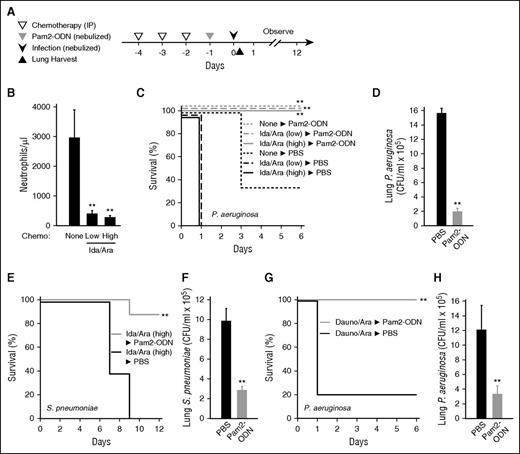
To recapitulate in mice the complex immunocompromise associated with remission induction therapy delivered to patients with AML, we tested dosing and schedule combinations of an anthracycline (Ida or Dauno) and an antimetabolite (Ara). We selected a regimen consisting of 3 consecutive days of intraperitoneal delivery of both agents (Figure 2A), based on induction of severe neutropenia without a high burden of spontaneous death due to marrow failure (Figure 2B; supplemental Figure 1, available on the Blood Web site). One day after the chemotherapy was completed, mice were treated with Pam2-ODN or sham and then challenged a day later with virulent bacterial pathogens. Despite neutropenic immune impairment, the mice were still robustly protected against otherwise lethal pneumonia caused by common clinical pathogens, including Gram-negative P. aeruginosa and Gram-positive S. pneumoniae (Figure 2C-H). The selection of anthracycline did not influence outcomes (Figures 1G and 2C,G). In each case, Pam2-ODN–enhanced survival was associated with significant reductions in the lung bacterial burden immediately after infection. As demonstrated with the slower progressing S. pneumoniae infection model, the survival advantage conferred by Pam2-ODN was evident >1 week after a single inhaled treatment (Figure 2E).18
Pam2-ODN protects mice against bacterial pneumonia despite cytotoxic chemotherapy. (A) Experimental schema. (B) Mouse circulating neutrophil counts after 3 days of treatment with idarubicin 33 mg/kg and cytarabine 1 mg/kg (low), idarubicin 50 mg/kg and cytarabine 1 mg/kg (high), or sham treatment with PBS (none). (C) Mouse survival of P. aeruginosa challenge (10 mL of 2 × 1010 CFU/mL bacterial suspension nebulized over 60 minutes) following the indicated treatments. (D) Lung bacterial burden of mice treated with high-dose idarubicin and cytarabine immediately after infection. (E) Mouse survival of S. pneumoniae challenge (10 mL of 2 × 1010 CFU/mL) following high-dose idarubicin and cytarabine treatment and inhaled treatment with Pam2-ODN or sham (PBS). (F) Lung bacterial burden immediately after infection. (G) Mouse survival of P. aeruginosa challenge (10 mL of 2 × 1010 CFU/mL) following high-dose daunorubicin and cytarabine treatment and inhaled treatment with Pam2-ODN or sham (PBS). (H) Lung bacterial burden immediately after infection. For survival experiments, N = 10 mice per group; for bacterial burden, N = 4 mice per group. *P < .005 vs no chemotherapy; **P < .05 vs mice treated with the same chemotherapy followed by PBS (sham) nebulization.
Pam2-ODN protects mice against bacterial pneumonia despite cytotoxic chemotherapy. (A) Experimental schema. (B) Mouse circulating neutrophil counts after 3 days of treatment with idarubicin 33 mg/kg and cytarabine 1 mg/kg (low), idarubicin 50 mg/kg and cytarabine 1 mg/kg (high), or sham treatment with PBS (none). (C) Mouse survival of P. aeruginosa challenge (10 mL of 2 × 1010 CFU/mL bacterial suspension nebulized over 60 minutes) following the indicated treatments. (D) Lung bacterial burden of mice treated with high-dose idarubicin and cytarabine immediately after infection. (E) Mouse survival of S. pneumoniae challenge (10 mL of 2 × 1010 CFU/mL) following high-dose idarubicin and cytarabine treatment and inhaled treatment with Pam2-ODN or sham (PBS). (F) Lung bacterial burden immediately after infection. (G) Mouse survival of P. aeruginosa challenge (10 mL of 2 × 1010 CFU/mL) following high-dose daunorubicin and cytarabine treatment and inhaled treatment with Pam2-ODN or sham (PBS). (H) Lung bacterial burden immediately after infection. For survival experiments, N = 10 mice per group; for bacterial burden, N = 4 mice per group. *P < .005 vs no chemotherapy; **P < .05 vs mice treated with the same chemotherapy followed by PBS (sham) nebulization.
Pam2-ODN protects chemotherapy-treated mice against fungal pneumonia.
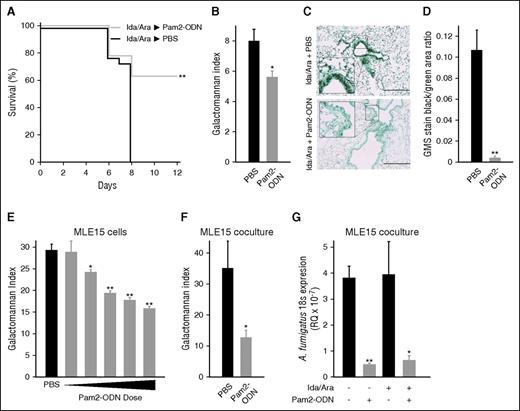
Ida/Ara-treated mice were similarly challenged with A. fumigatus, and were found to be significantly protected against lethal pneumonia by Pam2-ODN (Figure 3A). Twenty-four hours after infection, the fungal burden was significantly lower in Pam2-ODN–treated mice, as demonstrated by galactomannan index (Figure 3B), despite the fact that the galactomannan assay was not developed for use in lung homogenates and the process of lung disruption may reduce the observable intergroup difference by liberating galactomannan from nonviable organisms. Concordantly, GMS stain (Figure 3C-D) of simultaneously harvested lungs reveals a profound reduction in the fungal burden of Pam2-ODN–treated lungs. Similar to the bacterial studies, Pam2-ODN treatment of MLE15 cells in monolayer culture induced dose-dependent reductions in fungal burden (Figure 3E). This protective effect persisted when MLE15 cells were grown in coculture with FBL3 cells (Figure 3F) or in the presence of Ida/Ara chemotherapy (Figure 3G), whether assayed by culture galactomannan index or by quantitative real-time PCR for fungal 18s rRNA expression.
Pam2-ODN protects against fungal pneumonia. (A) Survival of mice treated with high-dose chemotherapy, followed by nebulized Pam2-ODN or PBS treatment 24 hours before A. fumigatus infection (10 mL of 3 × 108 conidia/mL suspension nebulized over 60 minutes). (B) Galactomannan indices and (C) histopathology with GMS stain of lungs harvested 24 hours after the infection in A. (D) Quantification of the area staining positive for A. fumigatus in C. (E) Galactomannan indices of MLE15 cell monolayer cultures 24 hours after A. fumigatus infection (3 × 103 conidia per well) following the indicated treatments. (F) Galactomannan indices of MLE15 cells grown in coculture with FBL3 cells, 24 hours after A. fumigatus infection (1 × 103 conidia per transwell insert). (G) Quantitative PCR for A. fumigatus 18s rRNA expression relative to host 18s rRNA expression (RQ) 24 hours after infection in the presence or absence of chemotherapy. N = 10 mice per group for survival experiments; N = 4 mice per group for fungal burden assays. *P < .05 vs PBS treated; **P < .005 vs PBS treated.
Pam2-ODN protects against fungal pneumonia. (A) Survival of mice treated with high-dose chemotherapy, followed by nebulized Pam2-ODN or PBS treatment 24 hours before A. fumigatus infection (10 mL of 3 × 108 conidia/mL suspension nebulized over 60 minutes). (B) Galactomannan indices and (C) histopathology with GMS stain of lungs harvested 24 hours after the infection in A. (D) Quantification of the area staining positive for A. fumigatus in C. (E) Galactomannan indices of MLE15 cell monolayer cultures 24 hours after A. fumigatus infection (3 × 103 conidia per well) following the indicated treatments. (F) Galactomannan indices of MLE15 cells grown in coculture with FBL3 cells, 24 hours after A. fumigatus infection (1 × 103 conidia per transwell insert). (G) Quantitative PCR for A. fumigatus 18s rRNA expression relative to host 18s rRNA expression (RQ) 24 hours after infection in the presence or absence of chemotherapy. N = 10 mice per group for survival experiments; N = 4 mice per group for fungal burden assays. *P < .05 vs PBS treated; **P < .005 vs PBS treated.
Pam2-ODN does not exacerbate tumor burden
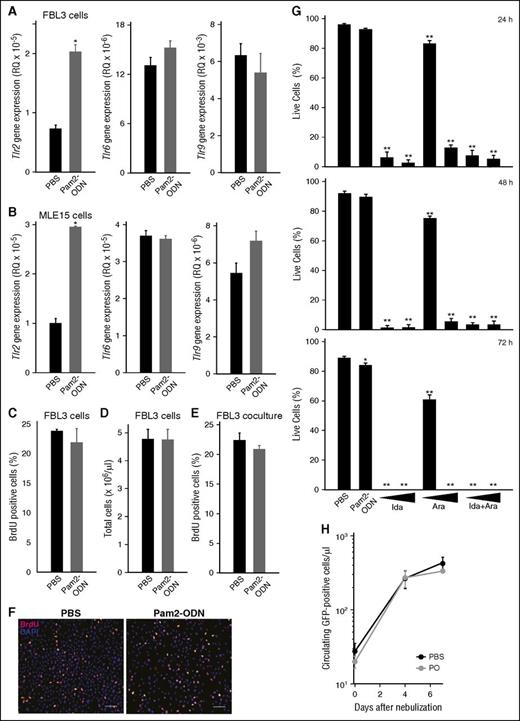
Lung epithelial cells, AML cells, and high-risk MDS cells are known to express TLRs. To exclude the possibility that a TLR-based therapy could induce proliferation of tumor cells or otherwise interfere with leukemia treatment, we directly assessed the effect of Pam2-ODN on FBL3 cells. As expected, quantitative PCR analysis confirmed TLR gene expression by both FBL3 and MLE15 cells. Both cell types revealed induction of Tlr2 gene expression, but not Tlr6 or Tlr9, following Pam2-ODN treatment (Figure 4A-B). However, Pam2-ODN did not induce FBL3 cell replication based on BrdU staining or manual cell counts, whether in monolayer or in coculture with MLE15 cells (Figure 4C-F). Alternately, idarubicin and cytarabine caused profound killing of FBL3 cells that progressed over 72-hour exposure, whereas Pam2-ODN had little effect on FBL3 cells survival in culture (Figure 4G).
Pam2-ODN does not influence tumor burden. Quantitative PCR for TLR genes 4 hours after treatment with Pam2-ODN or PBS of (A) FBL3 cells or (B) MLE15 cells. (C) BrdU-positive FBL3 cells 24 hours after the indicated treatments. (D) Total FBL3 cells counts by hemacytometer 24 hours after treatment. (E) BrdU-positive FBL3 cells 24 hours after treatment when grown in coculture with MLE15 cells. (F) BrdU staining of FBL3 cells. Scale bar, 100 µm. (G) Live FBL3 cells determined by Trypan blue exclusion at the indicated time points after treatment. (H) Flow cytometry for GFP-positive cells in mouse blood after treatment with Pam2-ODN or PBS. All data represent ≥3 experiments. N = 4 mice per group. *P < .005, **P < .0001.
Pam2-ODN does not influence tumor burden. Quantitative PCR for TLR genes 4 hours after treatment with Pam2-ODN or PBS of (A) FBL3 cells or (B) MLE15 cells. (C) BrdU-positive FBL3 cells 24 hours after the indicated treatments. (D) Total FBL3 cells counts by hemacytometer 24 hours after treatment. (E) BrdU-positive FBL3 cells 24 hours after treatment when grown in coculture with MLE15 cells. (F) BrdU staining of FBL3 cells. Scale bar, 100 µm. (G) Live FBL3 cells determined by Trypan blue exclusion at the indicated time points after treatment. (H) Flow cytometry for GFP-positive cells in mouse blood after treatment with Pam2-ODN or PBS. All data represent ≥3 experiments. N = 4 mice per group. *P < .005, **P < .0001.
As FBL3 cells are syngeneic to C57BL/6J mice,34 they engraft following tail vein injection without a conditioning regimen. FBL3 cells also contain a GFP construct that is stochastically expressed, allowing for comparisons of tumor burden by flow cytometry of blood samples. Using this approach, we found no increase in GFP-positive cells at any time point caused by Pam2-ODN treatment (Figure 4H), indicating that Pam2-ODN treatment had no impact on tumor engraftment or population size in vivo.
Inducible resistance is not impaired by the presence of leukemia cells or chemotherapy
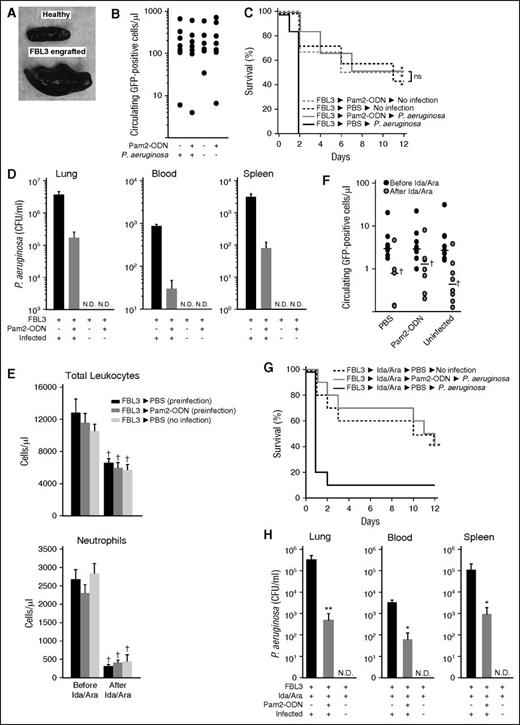
To recapitulate severely ill leukemia patients, mice injected with FBL3 cells were allowed to heavily engraft until the group demonstrated evidence of pathophysiology, such as reduced mobility, impaired feeding, or splenomegaly (Figure 5A). Flow cytometry for circulating GFP-positive cells was used to allocate mice to treatment groups in a manner that ensured similar tumor burden at the time of the infectious challenge (Figure 5B). In multiple trials, we observed that 15% to 25% of mice typically died due to effects of overwhelming leukemia prior to group allocation (supplemental Figure 2). FBL3-engrafted mice were allocated to 3 groups. Two groups received either nebulized Pam2-ODN or PBS, followed by P. aeruginosa challenge. A third group received nebulized PBS but was not infected, allowing assessment of the rate of death due to leukemia alone. In our prior studies, all mice that succumbed to P. aeruginosa pneumonia died less than 6 days after infection. In this study, we observed for twice that period to allow observation of the leukemia-related survival curve. As shown in Figure 5C, all sham-treated mice died within 3 days of the infectious challenge. Mice that received Pam2-ODN prior to infection demonstrated significantly improved survival of P. aeruginosa challenge over the PBS-treated mice, and their survival was statistically indistinguishable from leukemic mice that had not been infected at all. Among the mice that died during the observation period, sham-treated mice demonstrated much higher bacterial burdens in the lung, blood, and spleen. Not surprisingly, the uninfected mice did not grow any bacteria from any tested source (Figure 5D).
Pam2-ODN protects against pneumonia in the presence of leukemia and chemotherapy. (A) Photograph of spleens. (B) GFP-positive cells at group allocation. (C) Survival of FBL3-engrafted mice following the indicated treatments, with or without. P. aeruginosa infection (10 mL of 2 × 1010 CFU/mL). (D) Bacterial burdens of the indicated organs at the time of death of mice from C. (E) Circulating leukocytes (top) and neutrophils (bottom) of FBL3-engrafted mice before and after high-dose chemotherapy at the time of group allocation. (F) GFP-positive cells for the samples in E. (G) Survival of FBL3-engrafted, chemotherapy-treated mice following the indicated treatments, with or without P. aeruginosa infection (10 mL of 2 × 1010 CFU/mL). (H) Bacterial burdens of the indicated organs at the time of death of mice from G. (A-D) and (E-H) each present a single experiment that is representative of ≥3 replicates. *P < .05 vs FBL3→PBS→P. aeruginosa. **P < .005 vs FBL3→PBS→P. aeruginosa. †P < .05 vs before chemotherapy.
Pam2-ODN protects against pneumonia in the presence of leukemia and chemotherapy. (A) Photograph of spleens. (B) GFP-positive cells at group allocation. (C) Survival of FBL3-engrafted mice following the indicated treatments, with or without. P. aeruginosa infection (10 mL of 2 × 1010 CFU/mL). (D) Bacterial burdens of the indicated organs at the time of death of mice from C. (E) Circulating leukocytes (top) and neutrophils (bottom) of FBL3-engrafted mice before and after high-dose chemotherapy at the time of group allocation. (F) GFP-positive cells for the samples in E. (G) Survival of FBL3-engrafted, chemotherapy-treated mice following the indicated treatments, with or without P. aeruginosa infection (10 mL of 2 × 1010 CFU/mL). (H) Bacterial burdens of the indicated organs at the time of death of mice from G. (A-D) and (E-H) each present a single experiment that is representative of ≥3 replicates. *P < .05 vs FBL3→PBS→P. aeruginosa. **P < .005 vs FBL3→PBS→P. aeruginosa. †P < .05 vs before chemotherapy.
As the infectious susceptibility of leukemia patients derives from both the disease and its treatment, we exposed mice to both elements prior to testing the protective effects of Pam2-ODN. After engraftment with FBL3 cells, mice were treated with Ida (1 mg/kg, intraperitoneally) and Ara (50 mg/kg, intraperitoneally) for 3 days, and then the mice were allocated to 3 groups based on FBL3-GFP counts to ensure similar disease burden between groups. Chemotherapy treatment resulted in significant reductions in total leukocytes and GFP-positive cells and caused severe neutropenia, although there were no significant intergroup differences by any measured parameter (Figure 5E-F). No impairment of inducible resistance was observed in the presence of leukemia cells and cytotoxic chemotherapy. Pam2-ODN treatment significantly improved infectious survival over sham treatment, and mice that received Pam2-ODN treatment before infectious challenge demonstrated identical survival to mice that had not been infected at all (Figure 5G). Once again, the sham-treated mice had significantly higher bacterial burdens at the time of death than did Pam2-ODN–treated mice (Figure 5H).
Inducible resistance persists in the presence of human AML.
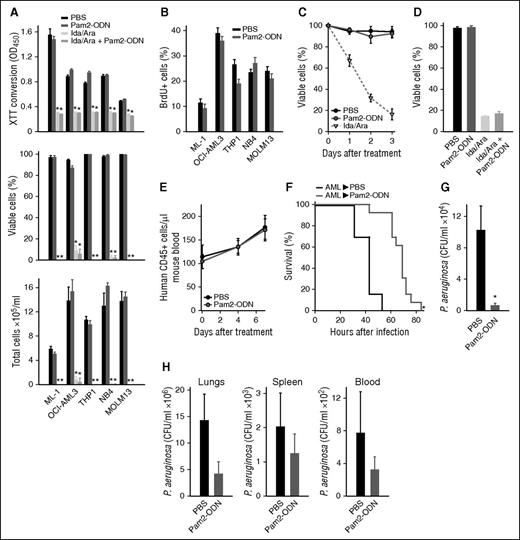
As FBL3 cells are mouse derived and formally considered erythroleukemia cells, it was important to confirm that no untoward interactions occurred between Pam2-ODN and human AML cells. Whether measured by XTT conversion, trypan blue exclusion or hemacytometer counts, Pam2-ODN treatment had no effect on the robust cytotoxic effect of Ida/Ara on any of 5 tested human AML cell lines (Figure 6A), nor did Pam2-ODN have any effect on human AML cell line proliferation, as measured by BrdU staining (Figure 6B). Congruently, Pam2-ODN had no effect on cell survival or chemotherapy-dependent killing of primary human AML cells (Figure 6C-D).
Pam2-ODN protects against pneumonia in the presence of human AML. (A) Viability of human AML cell lines 72 hours after the indicated treatments, assessed by XTT conversion (top), trypan blue exclusion (middle), and hemacytometer counts (bottom). (B) Influence of Pam2-ODN on BrdU staining in human AML cell lines. (C) Trypan blue exclusion of primary human AML cells following the indicated treatments. (D) Trypan blue exclusion of primary human AML cells 72 hours after the indicated treatments. (E) Circulating human AML cells in engrafted NSG mice following treatment with Pam2-ODN or PBS. (F) Survival of primary human AML-engrafted NSG mice after P. aeruginosa infection (10 mL of 9 × 109 CFU/mL) following treatment with Pam2-ODN or PBS. (G) Bacterial burdens of lungs harvested immediately after infection in F. (H) Bacterial burdens of the indicated organs at the time of death of mice from F. *P < .05 vs PBS treated, **P < .005 vs PBS treated.
Pam2-ODN protects against pneumonia in the presence of human AML. (A) Viability of human AML cell lines 72 hours after the indicated treatments, assessed by XTT conversion (top), trypan blue exclusion (middle), and hemacytometer counts (bottom). (B) Influence of Pam2-ODN on BrdU staining in human AML cell lines. (C) Trypan blue exclusion of primary human AML cells following the indicated treatments. (D) Trypan blue exclusion of primary human AML cells 72 hours after the indicated treatments. (E) Circulating human AML cells in engrafted NSG mice following treatment with Pam2-ODN or PBS. (F) Survival of primary human AML-engrafted NSG mice after P. aeruginosa infection (10 mL of 9 × 109 CFU/mL) following treatment with Pam2-ODN or PBS. (G) Bacterial burdens of lungs harvested immediately after infection in F. (H) Bacterial burdens of the indicated organs at the time of death of mice from F. *P < .05 vs PBS treated, **P < .005 vs PBS treated.
To test the effectiveness of Pam2-ODN in the setting of human AML, NSG mice were engrafted with primary human AML cells. Engraftment was confirmed by flow cytometry for human CD45-positive cells in mouse blood, and we confirmed that Pam2-ODN did not influence the tumor burden in vivo (Figure 6E). Similar to the FBL3 experiments, mice were allocated to comparable groups based on human CD45-positive cell counts and then treated with Pam2-ODN or PBS prior to challenge with P. aeruginosa. Despite their profound immunodeficiency, Pam2-ODN–treated mice lived significantly longer after infection than did sham-treated mice (Figure 6F). As demonstrated in all prior studies, this protection was associated with a significant reduction in lung bacterial burden immediately after infection (Figure 6G). At the time of death, there was also a trend toward lower bacterial burdens in the lungs, blood, and spleens of Pam2-ODN–treated mice (Figure 6H).
Discussion
Lower respiratory tract infections frequently complicate the management of AML and MDS. In addition to exacting unacceptably high attributable mortality, pneumonia in leukemia patients also substantially increases the consumption of medical resource and precludes the use of potentially curative myeloablative chemotherapies. Thus, enhanced control of pneumonia could substantively improve clinical outcomes of patients with acute leukemia. In clinical practice, the importance of pneumonia control is well established, as evinced by the widespread use of prophylactic antibiotics in patients with hematologic malignancies and their frequent housing in protected environments. Yet, despite these extensive efforts, leukemia patients continue to succumb to pneumonia at an alarming rate.
The susceptibility of leukemia patients to pneumonia is multifaceted. Chemotherapy-associated neutropenia, although both the most frequent and important risk factor for pneumonia in this population, is only one of many immune defects observed in AML/MDS patients.15-17 Because of the complex leukocyte defects observed in these patients, our laboratory has sought alternate means to protect them from pneumonia during periods of peak vulnerability. We demonstrate here that the strategy of therapeutically manipulating responses from epithelial cells is efficacious in protecting mice from pneumonia, despite the presence of leukemia cells and/or common leukemia therapy.
Consistent with our prior reports,25,27 mice remain protected against pneumonia by Pam2-ODN inhalation despite severe neutropenia, whereas the protection is completely lost when TLR signaling is selectively disrupted in the lung epithelium. Even in the absence of any leukocyte contributions, we demonstrate that isolated lung epithelial cells can generate active antimicrobial responses, and these responses are not abrogated by interaction with leukemia cells or cytotoxic therapies.
Previous reports have indicated that pathogen-triggered responses from lung epithelial cells are less impaired by immunosuppressive therapies than are those from myeloid cells.23,24 These observations, along with the low replicative rate of lung epithelial cells,35-37 prompted us to hypothesize that lung epithelial cells would be relatively tolerant of leukemia chemotherapy. Although reports exist about clinical lung toxicities of leukemia therapy,38 there is scant literature regarding the molecular effects of myeloablative therapies on lung epithelial cells. Thus, our findings that no tested chemotherapy agents impaired epithelial cell antimicrobial function or survival are notable. It might have been reasonably predicted that stimulation with TLR agonist might compound chemotherapy-induced toxicity on lung epithelial cells, but no such additive toxicity was observed. This is, perhaps, less surprising when considering that others have reported that TLR stimulation can protect against such toxic therapies as radiation.39
Another important observation of this study is the absence of untoward interactions of Pam2-ODN and leukemia cells. It was not previously known whether the presence of leukemia cells might negatively impact the host response to Pam2-ODN, but we found no impairment of inducible resistance in the presence of human or mouse leukemia cells. Given their expression of TLRs, it was also not known whether the Pam2-ODN might cause expansion of the tumor population. The current work demonstrates no evidence that Pam2-ODN treatment of primary or transformed leukemia cells increases their replicative rate nor their total number. Further, Pam2-ODN did not impair killing of leukemia cells by the chemotherapeutic agents.
Although we demonstrate no unfavorable interactions between Pam2-ODN, leukemia cells or leukemia treatments, the present study is also remarkable for the positive findings of anti-pneumonia efficacy. Recent series report microbiologically documented respiratory infections in 14% to 60% of AML patients, varying with treatment regimen.14,40-42 The most commonly isolated pathogens in this population are the Gram-negative bacterium P. aeruginosa, the Gram-positive bacterium S. pneumoniae and the endemic fungus A. fumigatus.40-45 Here, we demonstrate robust protection against all 3 frequently documented causes of pneumonia. The induction of broad activation of antimicrobial responses is notable, both because leukemia patients are frequently susceptible to pneumonia caused by uncommon pathogens and because the pathogen is often unknown due to inability to obtain a sample, culture-suppressive effects of ongoing antibiotic therapy or technical inability to detect obscure pathogens.15-17 Thus, not only do the current studies suggest that inducible epithelial resistance is likely to persist in profoundly immunocompromised, neutropenic leukemia patients, but they also indicate that this strategy is likely to benefit patients in a manner that does not depend on pathogen identification. This broad protection bears some conceptual similarity to trained innate immunity in monocytes, macrophages, and natural killer cells.46 However, although we observe renewed protection with repetitive Pam2-ODN treatments (ie, no tachyphylaxis),18,27 there is not clear evidence of a memory response to subsequent pathogen challenges, defining these as distinct phenomena. Moreover, the antimicrobial response kinetics do not indicate a classical priming event.
Interestingly, survival of fungal challenges following Pam2-ODN treatment in the current studies appears modestly less than that observed in mice that are challenged with bacteria after Pam2-ODN treatment. Although this may reflect a slightly lesser susceptibility of conidia to the Pam2-ODN–induced host defense molecules, it is also suspected that at least some of the late deaths reflect chemotherapy-related marrow failure (at time points beyond the observation period of bacterial studies), rather than infectious deaths, given the profound Pam2-ODN–induced reductions in pathogen burdens. Although the current study does not address the therapeutic use of Pam2-ODN, our prior work reveals a survival benefit of postinfection Pam2-ODN delivery in slower progressing infection (due to pathogen characteristics or inoculum size).18,25,33 Thus, use against fungal pneumonia in high-risk populations, where it is frequently difficult to ascertain the timing of exposure, may prove eventually beneficial.
These data reveal profound Pam2-ODN–induced protection against pneumonia caused by a wide range of pathogens, despite the complex immune impairments associated with leukemia and leukemia therapy. Given the principal reliance on lung epithelial cells, rather than dysfunctional leukocytes, it may be reasonable to infer that this protection may also be achievable in patients with other hematologic malignancies or in those who have received stem cell transplants. Taken together, these findings may suggest a means to protect extremely vulnerable patients against a common and lethal complication, thereby improving outcomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) grant R01 HL117976, NIH NHLBI Office of the Director grant DP2 HL123229 (S.E.E.), NIH National Cancer Institute (NCI) support grant P30 CA016672, and NIH NCI Leukemia Specialized Programs of Research Excellence grant P50 CA100632 to the MD Anderson Cancer Center.
Authorship
Contribution: M.M.L.-J., H.H.W., V.V.K., and M.J.T. designed experiments, performed experiments, and performed analyses; P.A.Z.-M. designed experiments and performed analyses; and S.E.E. designed experiments, performed analyses, and wrote the manuscript.
Conflict-of-interest disclosure: M.J.T. and S.E.E. are authors on US patent 8 883 174 entitled “Stimulation of Innate Resistance of the Lungs to Infection with Synthetic Ligands.” M.J.T. and S.E.E. own stock in Pulmotect, which holds the commercial options on these patent disclosures. M.M.L.-J., H.H.W., V.V.K., and P.A.Z.-M. declare no competing financial interests.
Correspondence: Scott E. Evans, 1515 Holcombe Blvd, Unit 1100, Houston, TX 77030; e-mail: seevans@mdanderson.org.