Like children alive when their parents have not been born, there is a population of natural killer (NK) cells which appear to be self-renewing, according to 2 articles in this issue of Blood. Corat et al and Schlums et al take us closer to this mystery by answering the question: is ongoing production from hematopoietic stem and progenitor cells (HSPCs) required to maintain NK-cell homeostasis in humans?1,2
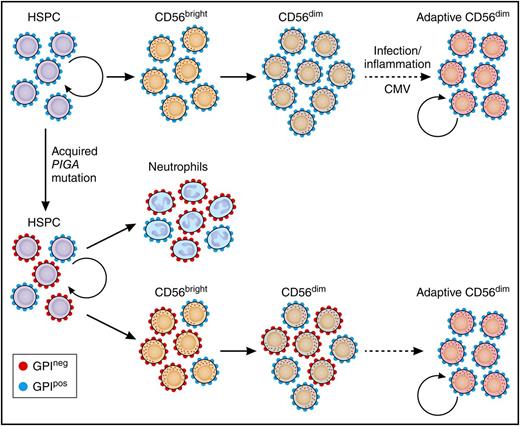
Somatic PIGA in HSPCs reveals longevity of adaptive NK cells. Normal HSPCs give rise to CD56bright NK cells that further develop to canonical CD56dim NK cells. Adaptive, CD56dim NK cells derive from canonical CD56dim NK-cell precursors in response to environmental stimuli. Patients with an acquired PIGA mutation exhibit mixed chimerism of GPIpos and GPIneg myeloid cells (represented here by neutrophils), reflecting the ongoing clonal contribution of PIGA-mutated self-renewing HSPCs. CD56bright NK cells develop proportionally from GPIpos and GPIneg HSPCs similar to myeloid cells. However, GPIneg cells are underrepresented in the CD56dim NK population, most prominently in CD56dim NK cells with adaptive phenotype. The persistence of GPIpos NK cells in PNH patients supports the notion of long-lived NK-cell adaptive NK cells with self-renewal capacity similar to memory T cells. See Figure 4 in the article by Corat et al that begins on page 1940. Professional illustration by Patrick Lane, ScEYEnce Studios.
Somatic PIGA in HSPCs reveals longevity of adaptive NK cells. Normal HSPCs give rise to CD56bright NK cells that further develop to canonical CD56dim NK cells. Adaptive, CD56dim NK cells derive from canonical CD56dim NK-cell precursors in response to environmental stimuli. Patients with an acquired PIGA mutation exhibit mixed chimerism of GPIpos and GPIneg myeloid cells (represented here by neutrophils), reflecting the ongoing clonal contribution of PIGA-mutated self-renewing HSPCs. CD56bright NK cells develop proportionally from GPIpos and GPIneg HSPCs similar to myeloid cells. However, GPIneg cells are underrepresented in the CD56dim NK population, most prominently in CD56dim NK cells with adaptive phenotype. The persistence of GPIpos NK cells in PNH patients supports the notion of long-lived NK-cell adaptive NK cells with self-renewal capacity similar to memory T cells. See Figure 4 in the article by Corat et al that begins on page 1940. Professional illustration by Patrick Lane, ScEYEnce Studios.
What is the answer? NK cells, comprising 2% to 10% of our lymphocyte population, are important first-line killers of virally infected or malignant cells. NK cells are thought to develop directly from bone marrow HSPCs, maturing in the periphery from CD56bright NK cells to cytotoxic CD56dim cells. When they come across a malignant or virally infected cell, mature CD56dim NK cells kill via release of granules containing perforin and granzyme, production of cytokines interferon γ (IFN-γ) and tumor necrosis factor α, and engagement of death receptors. The signal to kill occurs via direct contact of NK surface receptors with a target cell, via stimulation with humoral cytokines including interleukin-12 (IL-12) and IL-18, or engagement of the low-affinity FcR CD16.
Typical NK cells (the authors use the Greek-derived word “canonical” to describe these) have a half-life of 14 days. More recently, a new population of “adaptive” NK cells has been described in the context of cytomegalovirus (CMV) infection or response to vaccines. Although the mechanism for memory maintenance is unclear given the lack of rearranged antigen receptors, these cells can maintain immunological memory long-term similar to T cells.3-5 Adaptive NKs show diminished responses to IL-12 and IL-18 but retain the ability to degranulate and produce cytokines in response to FcR CD16 engagement.6,7 Schlums et al and Corat et al provide evidence for long-lived NK cells of the adaptive phenotype. One of the striking features of these NK cells is that they do not require ongoing production from HSPCs; they are, in a way, self-renewing.
Recent evidence for self-renewing NK cells in macaques by Dunbar et al using bar-coding studies showed limited clonal overlap between CD56dim NK and other hematopoietic cells including CD56bright NK cells.8 Clinical and murine adoptive transfer studies have also recently provided evidence for in vivo proliferation and persistence of mature NK cells.9,10 The authors take this 1 step further in human patients by making innovative use of naturally occurring disease models to identify hematopoietic progenitors and their progeny and show that there is a population of adaptive NKs circulating which are maintained independently from HSPCs for many years.
Schlums et al use the model of GATA-2. Patients with autosomal-dominant loss-of-function mutations in GATA-2 (a transcription factor required for HSPC survival and proliferation) have high mortality. They generally lose their hematopoietic progenitors, resulting in a progressive loss of monocytes, dendritic cells, and B and NK cells, and present with severe infections from mycobacteria, human papillomavirus and herpesviruses, cytopenias, myelodysplastic syndrome, or acute myeloid leukemia. In GATA-2–mutated patients without NK progenitors, or in whom hematopoietic progenitors have been lost, we expect no mature NK cells, yet Schlums et al detect NK cells in the peripheral blood of these individuals. They are, however, NK with a twist, having this atypical phenotype recently described as “adaptive” that resembles T cells in some ways. Unlike the typical mature CD56dim NK cells described above, adaptive NK cells have a particular phenotype (PLZFlow, FcεRγ−, SYK−, EAT-2−). In vitro functional studies prove these long-lived NK cells can produce IFN-γ in response to FcR engagement but not following IL-12 and IL-18 stimulation, consistent with previous descriptions of adaptive NK cells.
Corat et al shed yet more light on the mystery of orphan NK cells using the model of paroxysmal nocturnal hemoglobinuria (PNH). Patients with this disease have somatic X-linked mutations in PIGA which encode an enzyme essential for the synthesis of glycosylphosphatidylinositol (GPI) cell membrane proteins, including those protecting cells from complement-mediated red cell lysis. Patients can have stable levels of GPI-positive (GPIpos) and GPI-negative (GPIneg) red cells and neutrophils for many years, reflecting ongoing output from both mutated and unmutated HSPCs. In PNH, offspring of mutated and unmutated HSPCs can be identified by flow cytometric quantification of their surface GPI anchors. Knowing neutrophils mature directly from HSPCs, the authors use neutrophil GPI expression as an indirect measure of what is happening at the progenitor level in real time. If NK cells were all produced continuously from the same pool of HSPCs, we would expect the proportion of GPIneg NK cells to be similar to the proportion of GPIneg neutrophils. However, we find that circulating NK populations, particularly adaptive NK, have only small proportions of GPIneg cells and NK cells from clones still normally expressing GPI persist longer than would be expected, suggesting the circulating GPIpos NK cells are propagating independently of HSPCs (see figure). The lower proportions of GPIneg NK cells in the circulation are in fact close to that of T cells which we know can propagate independently, suggesting NK cells do likewise.
The concept of self-renewing NK cells is very recent and characterizing them in humans is novel. These articles also make unique use of naturally occurring stem cell mutations to track progeny, which in these days of bar coding and cell trackers, is elegantly simple. These articles challenge current thinking about NK-cell development.
But what does all of this mean? What is the clinical significance of these longer-lived NK cells? Can they be harnessed to improve longevity of NK cellular therapies? Certainly there is potential for the detailed characterization provided in these articles to be exploited in this way. With a typical 2-week half-life, NK-cell therapy is a short-lived anticancer strategy that can be repeated if necessary. Strategies are under way to improve the proliferation and persistence of adoptively transferred NK-cell therapy using cytokines such as IL-15, but selecting or specifically expanding long-lived self-renewing NK cells to transfer could avoid the potential side effects of this and provide a pool of NK cells ready to respond to relapse. Further questions remain as to whether these new NK cells can kill tumor or virally infected cells and ensure they are not “exhausted” cells. If fully functional, as recent data suggest,11 the findings presented could potentially open the door to a new era of long-lived NK-cell therapies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal