Key Points
GATA-2 expression is restricted to hematopoietic stem and progenitor cells, leading to NK-cell progenitor deficiency in GATA2 patients.
A long-lived or self-renewing pool of adaptive NK cells can persist in patients with GATA2 mutation.
Abstract
Heterozygous GATA2 mutation is associated with immunodeficiency, lymphedema, and myelodysplastic syndrome. Disease presentation is variable, often coinciding with loss of circulating dendritic cells, monocytes, B cells, and natural killer (NK) cells. Nonetheless, in a proportion of patients carrying GATA2 mutation, NK cells persist. We found that peripheral blood NK cells in symptomatic patients uniformly lacked expression of the transcription factor promyelocytic leukemia zinc finger (PLZF), as well as expression of intracellular signaling proteins FcεRγ, spleen tyrosine kinase (SYK), and EWS/FLI1-Activated Transcript 2 (EAT-2) in a variegated manner. Moreover, consistent with an adaptive identity, NK cells from patients with GATA2 mutation displayed altered expression of cytotoxic granule constituents and produced interferon-γ upon Fc-receptor engagement but not following combined interleukin-12 (IL-12) and IL-18 stimulation. Canonical, PLZF-expressing NK cells were retained in asymptomatic carriers of GATA2 mutation. Developmentally, GATA-binding protein-2 (GATA-2) was expressed in hematopoietic stem cells, but not in NK-cell progenitors, CD3−CD56bright, canonical, or adaptive CD3−CD56dim NK cells. Peripheral blood NK cells from individuals with GATA2 mutation proliferated normally in vitro, whereas lineage-negative progenitors displayed impaired NK-cell differentiation. In summary, adaptive NK cells can persist in patients with GATA2 mutation, even after NK-cell progenitors expire. Moreover, our data suggest that adaptive NK cells are more long-lived than canonical, immunoregulatory NK cells.
Introduction
Loss-of-function mutations in GATA2 are associated with an autosomal-dominant typically adult-onset syndrome, with variable clinical presentation yet high mortality.1,2 Patients may present with severe mycobacterial, papilloma virus, and herpes virus family infections, lymphedema, hypocellular bone marrow failure, or myelodysplastic syndrome (MDS) evolving to acute myeloid leukemia (AML).3-9 GATA-binding protein-2 (GATA-2) is a transcription factor required for hematopoietic stem and progenitor cell (HSPC) survival and proliferation.10,11 GATA-2 haploinsufficiency generally manifests in a progressive loss of monocytes, dendritic cells (DCs), B cells, and natural killer (NK) cells, leading to increased susceptibility to certain infections.3,4,12-14 Reduction of monocyte, B-cell, as well as CD4+ T-cell numbers is associated with symptomatic disease, whereas cytotoxic effector CD8+ T-cell numbers generally persist.1,2 Remarkably, an index case of selective NK-cell deficiency associated with severe herpes virus infections including varicella, cytomegalovirus (CMV), and herpes simplex virus (HSV)15 was later found to harbor a heterozygous GATA2 mutation.16 With respect to NK cells, GATA2 mutation is associated with a loss of CD3−CD56bright NK cells, whereas differentiated CD3−CD56dim NK cells curiously persist in some patients.1,16
NK cells are lymphocytes that act at the interface between innate and adaptive immunity.17 They can eradicate infected and neoplastic cells, as well as autologous activated immune cells, by targeted release of cytotoxic granules containing perforin and granzymes. Moreover, NK cells can relay signals to other immune cells, producing interferon-γ (IFN-γ) in response to target cells or combinations of exogenous cytokines such as interleukin-2 (IL-2), IL-12, IL-15, and IL-18.18,19 Besides GATA2, one other gene defect has hitherto been associated with human NK-cell deficiency. Autosomal-recessive missense mutations in MCM4, encoding a component of the minichromosome maintenance (MCM) complex essential for genomic DNA replication, are associated with selective loss of the CD3−CD56dim NK-cell subset accompanied by an increase in relative frequencies of peripheral blood CD3−CD56bright NK cells.20 The loss of mature, cytotoxic CD3−CD56dim NK cells in cases of MCM4 deficiency thus contrasts GATA-2 haploinsufficiency, where seemingly differentiated NK cells may persist in the absence of assumed precursor CD3−CD56bright NK cells.1,16 These and other observations21 thus question established NK-cell developmental relationships.22
Recent studies of murine CMV infection have revealed that NK cells responding to infection can expand and persist.23 Such cells display features of adaptive T and B cells, including lasting expansions of cells that can undergo robust recall responses. In humans, we and others have uncovered CMV-associated NK cells with epigenetic similarities to cytotoxic effector CD8+ T cells. These so-called adaptive NK cells display characteristic DNA methylation–dependent silencing of the transcription factor promyelocytic leukemia zinc (PLZF), as well as stochastic loss of expression of membrane proximal signaling molecules FcεRγ, spleen tyrosine kinase (SYK), or EWS/FLI1-activated transcript 2 (EAT-2).24,25 Importantly, revealing a dichotomy in NK-cell function, such adaptive NK cells have a markedly reduced capacity for immunoregulatory killing of autologous activated T cells and lack responsiveness to IL-12 and IL-18.24,26 Notably, experiments in mice and observations in humans indicate that adaptive NK cells provide protective immunity to CMV.23,27 In humans, they mediate strong Fc receptor–dependent effector functions,24,25 ostensibly contributing to cross-protective antibody-mediated immunity. The molecular mechanisms of adaptive NK-cell development and homeostasis are poorly understood.28
Here, we have explored NK-cell phenotype and function in asymptomatic and symptomatic individuals with GATA2 mutation. Remarkably, we find that NK cells persisting in symptomatic individuals uniformly display phenotypic and functional attributes of adaptive NK cells. The results provide clues to NK-cell ontogenetic relationships and raise questions regarding the pathogenesis of GATA-2 haploinsufficiency.
Methods
Blood samples, cells, and antibodies
Sample collection was carried out via protocols approved by the regional ethical review in Stockholm, Sweden as well as the institutional review boards in Newcastle upon Tyne, United Kingdom and the National Institutes of Health, Bethesda, MD. Written informed consent was obtained from all individuals. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Lymphoprep; Axis-Shield), cryopreserved, and resuspended in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum, l-glutamine, penicillin, and streptomycin; all Hyclone).
For cell lines and antibodies, see supplemental Methods (available on the Blood Web site).
Flow cytometry
For phenotypic analyses, PBMCs were surface stained with fluorochrome-conjugated antibodies as indicated and a fixable dead cell stain (Invitrogen), fixed in 2% formaldehyde (Polysciences) in phosphate-buffered saline, and permeabilized in 0.05% Triton X-100 (Sigma-Aldrich) in phosphate-buffered saline for intracellular staining. For functional analyses, lymphocytes were stimulated, surface stained with antibodies and a fixable dead cell stain, as previously described.24,29 In experiments measuring cytokine production, GolgiPlug (BD Biosciences) was added during stimulation. Flow cytometry data acquisition and analyses are detailed in supplemental Methods.
Transcription factor cloning and interaction studies
See supplemental Methods.
Ex vivo NK-cell expansions
See supplemental Methods.
Results
Predominance of NK cells lacking PLZF expression in patients with heterozygous GATA2 mutation
Previous reports of patients with heterozygous GATA2 mutation have described heterogeneity in NK-cell numbers, with some individuals having high frequencies of differentiated peripheral blood NK cells despite loss of less mature CD3−CD56bright cells.1,16 Sparked by the characterization of long-lived NK cells in mice,23 we hypothesized that residual NK cells in human patients with bone marrow failure might constitute adaptive cells. We analyzed 10 adult patients with GATA2 mutation and clinical manifestations, in addition to 3 asymptomatic adult carriers (Table 1). As expected, most patients had markedly elevated levels of circulating FLT-3 ligand, and markedly reduced but heterogeneous peripheral blood B-cell, monocyte, and NK-cell counts (Table 1). Relative to healthy controls, absolute numbers of CD3−CD56+/CD16+ NK cells among lymphocytes were low in the majority of patients and in all asymptomatic carriers (Figure 1A). The majority of patients displayed reduced frequencies of CD3−CD56bright NK cells, as previously described,1,16,30 whereas asymptomatic carriers maintained these cells (Figure 1B; supplemental Tables 1 and 2). Of note, 4 of 6 GATA2-mutated patients and asymptomatic carriers retaining CD3−CD56bright NK cells were CMV seronegative with the remaining 2 having unknown CMV status at the time of blood collection (Figure 1B).
GATA2 mutations, clinical characteristics, and immunological features of patients
| Identifier . | GATA2 cDNA . | Protein . | Age at onset, years . | HPV . | Myco . | URTI . | Lung . | PAP . | AI . | MDS . | Lympho-edema . | Cancer . | FLT-3 pg/mL . | CMV serology . | Cells/μL . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NK cells . | B cells . | Monocytes . | |||||||||||||||
| Patient 1 | c.599insG | p.G200fs | 60 | + | − | + | + | − | − | + | − | − | 2662 | ND | 380 | 114 | 260 |
| Patient 2 | c.803delG | 29 | + | + | + | + | + | − | − | − | − | 2096 | + | 124 | 16 | 20 | |
| Patient 3 | c.1017+572C>T | Intronic | 28 | + | − | − | − | − | − | + | − | − | 2274 | — | 10 | 7 | 20 |
| Patient 4 | c.1018-1G>T | p.D340-381 | 18 | + | − | + | − | − | + | − | − | − | 6874 | + | 21 | 71 | 4 |
| Patient 5 | c.1143+5G>A | Splicing | 21 | + | − | + | + | − | − | − | − | − | ND | + | 3 | 3 | 243 |
| Patient 6 | c.1186C>T | p.R396W | 7 | − | + | + | + | − | − | + | − | − | 1448 | + | 44 | 10 | 0 |
| Patient 7 | c.1186C>T | p.R396W | 18 | + | + | + | + | − | − | + | + | − | ND | − | 2 | 30 | 0 |
| Patient 8 | c.1187G>A | p.R396Q | 17 | + | − | − | + | − | − | + | − | − | ND | + | 21 | 34 | 0 |
| Patient 9 | c.1192C>T | p.R398W | ? | ND | − | ND | + | + | − | − | − | − | 3169 | ND | 103 | 26 | 800 |
| Patient 10 | c.1193G>A | p.R398Q | 29 | − | − | + | − | − | − | − | − | − | 81 | ND | 207 | 102 | 425 |
| Asympt. carrier 1 | c.1017+572C>T | Intronic | 61 | − | − | − | − | − | − | − | − | −* | 24 | − | 128 | 97 | 690 |
| Asympt. carrier 2 | c.1017+572C>T | Intronic | 52 | − | − | − | − | − | − | − | − | − | 34 | − | 106 | 149 | 510 |
| Asympt. carrier 3 | c.1193G>A | p.R398Q | 32 | − | − | − | − | − | − | − | − | − | 69 | ND | 10 | 140 | 684 |
| Identifier . | GATA2 cDNA . | Protein . | Age at onset, years . | HPV . | Myco . | URTI . | Lung . | PAP . | AI . | MDS . | Lympho-edema . | Cancer . | FLT-3 pg/mL . | CMV serology . | Cells/μL . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NK cells . | B cells . | Monocytes . | |||||||||||||||
| Patient 1 | c.599insG | p.G200fs | 60 | + | − | + | + | − | − | + | − | − | 2662 | ND | 380 | 114 | 260 |
| Patient 2 | c.803delG | 29 | + | + | + | + | + | − | − | − | − | 2096 | + | 124 | 16 | 20 | |
| Patient 3 | c.1017+572C>T | Intronic | 28 | + | − | − | − | − | − | + | − | − | 2274 | — | 10 | 7 | 20 |
| Patient 4 | c.1018-1G>T | p.D340-381 | 18 | + | − | + | − | − | + | − | − | − | 6874 | + | 21 | 71 | 4 |
| Patient 5 | c.1143+5G>A | Splicing | 21 | + | − | + | + | − | − | − | − | − | ND | + | 3 | 3 | 243 |
| Patient 6 | c.1186C>T | p.R396W | 7 | − | + | + | + | − | − | + | − | − | 1448 | + | 44 | 10 | 0 |
| Patient 7 | c.1186C>T | p.R396W | 18 | + | + | + | + | − | − | + | + | − | ND | − | 2 | 30 | 0 |
| Patient 8 | c.1187G>A | p.R396Q | 17 | + | − | − | + | − | − | + | − | − | ND | + | 21 | 34 | 0 |
| Patient 9 | c.1192C>T | p.R398W | ? | ND | − | ND | + | + | − | − | − | − | 3169 | ND | 103 | 26 | 800 |
| Patient 10 | c.1193G>A | p.R398Q | 29 | − | − | + | − | − | − | − | − | − | 81 | ND | 207 | 102 | 425 |
| Asympt. carrier 1 | c.1017+572C>T | Intronic | 61 | − | − | − | − | − | − | − | − | −* | 24 | − | 128 | 97 | 690 |
| Asympt. carrier 2 | c.1017+572C>T | Intronic | 52 | − | − | − | − | − | − | − | − | − | 34 | − | 106 | 149 | 510 |
| Asympt. carrier 3 | c.1193G>A | p.R398Q | 32 | − | − | − | − | − | − | − | − | − | 69 | ND | 10 | 140 | 684 |
A survey of GATA-2–related clinical features of the cohort is presented. Normal cellular ranges were: 126 to 729 NK cells per microliter; 61 to 321 B cells per microliter; and 300 to 820 monocytes per microliter.
+, Positive history; −, negative history; AI, autoimmunity (arthritis, panniculitis, or autoimmune cytopenia); Asympt., asymptomatic; Cancer, nonhematopoietic malignancy; cDNA, complementary DNA; HPV, persistent infection of hands, feet, or perineum with human papillomavirus; Lymph, lymphedema; Lung, loss of lung volume or transfer factor, 80% predicted, history of bronchiectasis, chronic bronchitis, >1 episode of pneumonia, radiologically or pathologically confirmed pulmonary alveolar proteinosis; MDS, WHO (refractory cytopenia with multilineage dysplasia); Myco, any history of mycobacterial infection; ND, not determined; PAP, pulmonary alveolar proteinosis; URTI, >3 episodes of recurrent bacterial sinusitis, otitis, or other upper respiratory tract infection.
Deceased.
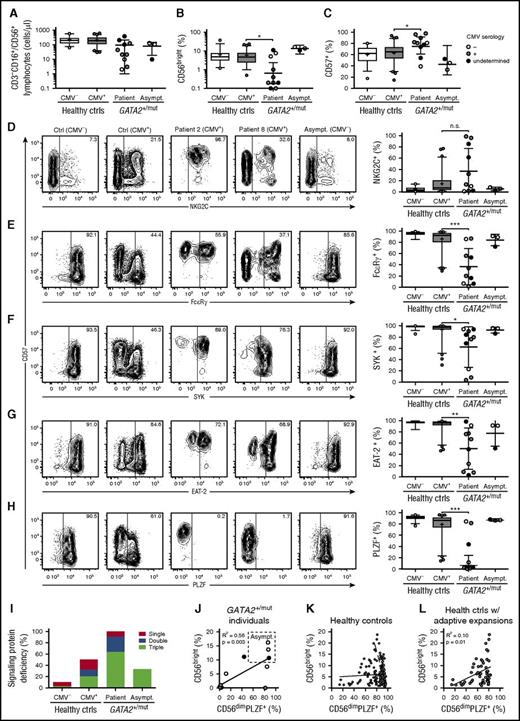
High prevalence of NK cells displaying lack of intracellular signaling protein and PLZF expression in patients with GATA2 mutation. PBMCs from CMV-seronegative (n = 69) and -seropositive (n = 127) healthy donors as well as patients (n = 10) and asymptomatic carriers (n = 3) with heterozygous GATA2 mutations were examined by flow cytometry. Graphs depict (A) the number of total NK cells as defined by CD3−CD56+/CD16+ cells in the lymphocyte gate, (B) the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population, and (C) the percentage of CD3−CD56dim NK cells expressing CD57. (D-H) Contour plots show CD3−CD56dim NK cells from representative CMV-seronegative and -seropositive healthy donors as well as 2 patients and 1 asymptomatic carrier with GATA2 mutations. Expression of CD57 is plotted against (D) NKG2C or the intracellular proteins (E) FcεRγ, (F) SYK, (G) EAT-2, and (H) PLZF. (D-H) Graphs depict the percentages of CD3−CD56dim NK cells expressing NKG2C, FcεRγ, SYK, EAT-2, or PLZF, as indicated. In the graphs (A-H), CMV serostatus for GATA2 patients and carriers is indicated as seronegative (open circles), seropositive (gray circles), or undetermined (black circles). (A-H) Boxes indicate 25th, 50th (median), and 75th percentiles, with a plus indicating the mean. For box plots, whiskers indicate 95% confidence intervals. Otherwise, error bars indicate mean with standard deviation (SD). P values were determined using the Student unpaired t test. *P < .05, **P < .01, and ***P < .001. (I) The overall frequency of healthy controls (ctrls, stratified on CMV seropositivity, or individuals with GATA2 mutations with significant, sizeable downregulations of 1, 2, or 3 of the signaling proteins FcεRγ, SYK, and EAT-2 is plotted. (J) In individuals with GATA2 mutation, the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population is plotted as a function of the frequency of PLZF-expressing CD3−CD56dim NK cells. (K) Among healthy controls, the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population is plotted as a function of the frequency of PLZF-expressing CD3−CD56dim NK cells. The CMV serostatus is indicated as seronegative (open circles) seropositive (gray circles). (L) Among healthy controls with sizeable adaptive NK-cell expansions as defined by PLZF deficiency, the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population is plotted as a function of the frequency of PLZF-expressing CD3−CD56dim NK cells. Healthy controls with sizeable adaptive NK-cell expansions were overwhelmingly CMV seropositive (gray circles).
High prevalence of NK cells displaying lack of intracellular signaling protein and PLZF expression in patients with GATA2 mutation. PBMCs from CMV-seronegative (n = 69) and -seropositive (n = 127) healthy donors as well as patients (n = 10) and asymptomatic carriers (n = 3) with heterozygous GATA2 mutations were examined by flow cytometry. Graphs depict (A) the number of total NK cells as defined by CD3−CD56+/CD16+ cells in the lymphocyte gate, (B) the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population, and (C) the percentage of CD3−CD56dim NK cells expressing CD57. (D-H) Contour plots show CD3−CD56dim NK cells from representative CMV-seronegative and -seropositive healthy donors as well as 2 patients and 1 asymptomatic carrier with GATA2 mutations. Expression of CD57 is plotted against (D) NKG2C or the intracellular proteins (E) FcεRγ, (F) SYK, (G) EAT-2, and (H) PLZF. (D-H) Graphs depict the percentages of CD3−CD56dim NK cells expressing NKG2C, FcεRγ, SYK, EAT-2, or PLZF, as indicated. In the graphs (A-H), CMV serostatus for GATA2 patients and carriers is indicated as seronegative (open circles), seropositive (gray circles), or undetermined (black circles). (A-H) Boxes indicate 25th, 50th (median), and 75th percentiles, with a plus indicating the mean. For box plots, whiskers indicate 95% confidence intervals. Otherwise, error bars indicate mean with standard deviation (SD). P values were determined using the Student unpaired t test. *P < .05, **P < .01, and ***P < .001. (I) The overall frequency of healthy controls (ctrls, stratified on CMV seropositivity, or individuals with GATA2 mutations with significant, sizeable downregulations of 1, 2, or 3 of the signaling proteins FcεRγ, SYK, and EAT-2 is plotted. (J) In individuals with GATA2 mutation, the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population is plotted as a function of the frequency of PLZF-expressing CD3−CD56dim NK cells. (K) Among healthy controls, the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population is plotted as a function of the frequency of PLZF-expressing CD3−CD56dim NK cells. The CMV serostatus is indicated as seronegative (open circles) seropositive (gray circles). (L) Among healthy controls with sizeable adaptive NK-cell expansions as defined by PLZF deficiency, the percentage of CD3−CD56bright NK cells within the total CD3−CD56+/CD16+ NK-cell population is plotted as a function of the frequency of PLZF-expressing CD3−CD56dim NK cells. Healthy controls with sizeable adaptive NK-cell expansions were overwhelmingly CMV seropositive (gray circles).
CMV-associated adaptive NK cells have been defined as CD57+NKG2C+ NK cells.31 Frequencies of CD57-expressing CD3−CD56dim NK cells were elevated in patients with GATA2 mutation, but normal in asymptomatic carriers (Figure 1C; supplemental Tables 1 and 2). Three patients displayed exceedingly high frequencies of NKG2C-expressing CD3−CD56dim NK cells (Figure 1D). However, several patients had low frequencies of NKG2C-expressing NK cells, including a CMV-seropositive patient. Asymptomatic carriers also displayed low frequencies of NKG2C-expressing NK cells, consistent with at least 2 of these individuals being CMV seronegative. As such, high proportions of adaptive CD57+NKG2C+ NK cells are found in some patients, but data do not strongly indicate an increased prevalence of adaptive NK cells in patients with GATA2 mutation.
We and others recently reported that CMV-associated human adaptive NK cells may lack expression of NK-cell signaling proteins FcεRγ, SYK, and EAT-2, whereas lack of the transcription factor PLZF generally defines such cells.24,25 Accordingly, adaptive NK cells do not necessarily express NKG2C or CD57.24,28 High frequencies of CD3−CD56dim NK cells variably lacking expression of the signaling molecules FcεRγ, SYK, or EAT-2 were a feature of patients with GATA2 mutation, but not asymptomatic carriers (Figure 1E-G). Strikingly, PLZF-expressing CD3−CD56dim NK cells were profoundly reduced or absent in 7 of 10 GATA2 patients (Figure 1H). In these patients, PLZF− subsets constituted the entire NK-cell compartment. In contrast, asymptomatic carriers displayed PLZF expression patterns comparable to healthy controls. Altogether, patients with GATA2 mutation displayed very high frequencies of NK cells lacking expression of 1 or more of the signature signaling proteins (Figure 1I). In individuals with GATA2 mutation, lack of PLZF expression in CD56dim NK cells correlated with loss of CD56bright NK cells and severe disease (Figure 1J). In contrast, PLZF expression among CD56dim NK cells and the frequency of CD56bright NK cells was not generally correlated among healthy controls (Figure 1K). However, when examined solely among individuals with sizeable expansions of adaptive NK cells, lack of PLZF expression among CD56dim NK cells correlated with reduced CD56bright NK-cell frequencies (Figure 1L). Together, these results suggest that NK cells with an adaptive phenotype may selectively persist in patients with GATA2 mutation.
Additional phenotypic features of adaptive NK cells in patients with GATA2 mutation
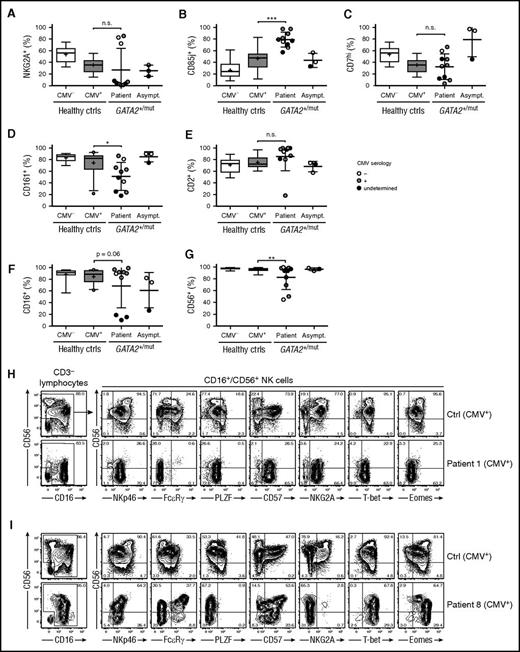
The NK cell-surface receptor phenotype has previously been examined in limited numbers of GATA2 patients.1,16 To substantiate the notion of persistent differentiated, adaptive NK cells, we examined the expression of NKG2A, CD85j, CD161, CD7, and CD2, variably expressed on canonical and adaptive NK cells.24 Patients exhibited dichotomous expression patterns for NKG2A, whereas asymptomatic carriers displayed frequencies of NKG2A+CD3−CD56dim NK cells similar to healthy controls (Figure 2A; supplemental Tables 1 and 2). Furthermore, expression of CD85j was elevated on CD3−CD56dim NK cells from patients, but not asymptomatic carriers (Figure 2B). Additionally, we observed significantly lower frequencies of CD3−CD56dim NK cells in patients expressing CD161 and high levels of CD7, as well as a trend of higher frequencies of cells expressing CD2 (Figure 2C-E; supplemental Figure 1). A single CMV-seropositive GATA2-mutated patient displayed NK cells with unusually low levels of CD2. These cells were CD3−CD56+/−CD16+/−CD57+FcεRγ−SYK−EAT-2+PLZF−CD2−NKG2A+NKG2C−CD85j+CD161+. In summary, peripheral blood NK cells from patients, but not asymptomatic carriers, expressed an adaptive NK cell-surface receptor phenotype. The enrichment of NK cells with an adaptive phenotype did not strictly correlate with CMV seropositivity.
Surface receptor expression patterns associated with adaptive NK cells in patients with GATA2 mutation. PBMCs from CMV-seronegative (n = 12) and -seropositive (n = 26) healthy donors as well as patients (n = 10) and asymptomatic carriers (n = 3) with heterozygous GATA2 mutations were examined by flow cytometry. Graphs depict the percentages of CD3−CD56dim/CD16+ NK cells expressing (A) NKG2A, (B) CD85j, (C) high levels of CD7, (D) CD161, (E) CD2, (F) CD16, and (G) CD56. CMV serostatus for GATA2 patients and carriers is indicated as seronegative (open circles), seropositive (gray circles), or undetermined (black circles). Boxes indicate 25th, 50th (median), and 75th percentiles, with a plus indicating the mean. For box plots, whiskers indicate 95% confidence intervals. Otherwise, error bars indicate mean with SD. P values were determined using the Student unpaired t test. *P < .05, **P < .01, and ***P < .001. (H-I) Contour plots show CD56 and CD16 expression on CD3− lymphocytes on representative CMV-seropositive healthy donors with adaptive NK-cell expansions as well as 2 selected patients with heterozygous GATA2 mutation and high frequencies of CD3−CD16+CD56− NK cells. Plots gated on CD3−CD16+/CD56+ NK cells depict CD56 vs NKp46, FcεRγ, PLZF, CD57, NKG2A, T-bet, and Eomes expression.
Surface receptor expression patterns associated with adaptive NK cells in patients with GATA2 mutation. PBMCs from CMV-seronegative (n = 12) and -seropositive (n = 26) healthy donors as well as patients (n = 10) and asymptomatic carriers (n = 3) with heterozygous GATA2 mutations were examined by flow cytometry. Graphs depict the percentages of CD3−CD56dim/CD16+ NK cells expressing (A) NKG2A, (B) CD85j, (C) high levels of CD7, (D) CD161, (E) CD2, (F) CD16, and (G) CD56. CMV serostatus for GATA2 patients and carriers is indicated as seronegative (open circles), seropositive (gray circles), or undetermined (black circles). Boxes indicate 25th, 50th (median), and 75th percentiles, with a plus indicating the mean. For box plots, whiskers indicate 95% confidence intervals. Otherwise, error bars indicate mean with SD. P values were determined using the Student unpaired t test. *P < .05, **P < .01, and ***P < .001. (H-I) Contour plots show CD56 and CD16 expression on CD3− lymphocytes on representative CMV-seropositive healthy donors with adaptive NK-cell expansions as well as 2 selected patients with heterozygous GATA2 mutation and high frequencies of CD3−CD16+CD56− NK cells. Plots gated on CD3−CD16+/CD56+ NK cells depict CD56 vs NKp46, FcεRγ, PLZF, CD57, NKG2A, T-bet, and Eomes expression.
In a previous report assessing the phenotype of NK cells in patients with GATA2 mutation, reduced CD16 expression levels on CD3−CD56dim NK cells was noted.1 Similarly, we found high frequencies of CD3−CD56dimCD16− NK cells in 3 of 10 patients with GATA2 mutation (Figure 2F). However, we also noted expansions of CD3−CD56−CD16+ cells expressing multiple NK-cell markers in patients with GATA2 mutation (Figure 2G-I). Expansions of such NK cells, which are generally poorly cytotoxic and demonstrate attenuated cytokine production, were originally described in settings of chronic viral infection such as HIV-1.32,33 Our observations thus indicate a high level of immune activation in several patients with GATA2 mutation, consistent with the severe persistent viral infections often observed upon development of cellular deficiencies.
Patients with GATA2 mutation display an altered cytotoxic granule content associated with adaptive NK-cell differentiation
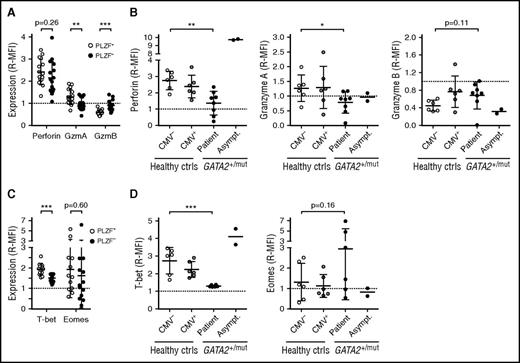
In terms of global DNA methylation, adaptive NK cells epigenetically approximate effector CD8+ αβT cells.24 Although allelic silencing of B and myeloid cell-signaling molecules imprints major functional changes, adaptive NK-cell differentiation is also accompanied by more subtle changes in protein expression. NK cells and cytotoxic T lymphocytes (CTLs) use the same machinery for target cell killing, yet relative expression levels of granule constituents differ.29 Investigating healthy CMV-seropositive donors (n = 16) with sizeable adaptive NK-cell populations by intracellular flow cytometry, we found that adaptive PLZF− as compared with canonical PLZF+ NK cells display lower expression of perforin and granzyme A and increased expression of granzyme B (Figure 3A; supplemental Figure 2A). As such, expression levels of granzyme A and B in adaptive NK cells approximated those of CD3+CD8+CD57+ CTLs. Expression of perforin and granzyme A was decreased and granzyme B increased in bulk CD3−CD56dim NK cells from patients, but not asymptomatic carriers (Figure 3B; supplemental Figure 2A), thus demonstrating a similar pattern of cytotoxic granule protein expression observed in adaptive NK cells.
Cytotoxic granule constituent and T-bet expression pattern resembling adaptive NK cells in patients with GATA2 mutation. PBMCs, healthy donors, and individuals with heterozygous GATA2 mutations were examined for expression of cytotoxic granule constituents perforin, granzyme A (GzmA), and granzyme B (GzmB) as well as the transcription factors T-bet and Eomes by intracellular flow cytometry. Expression is shown as median fluorescence intensity relative to (R-MFI) CD3+CD8+CD57+ T cells in the same sample. (A) The graph depicts expression of perforin, granzyme A and B in conventional PLZF+ (open circles) and adaptive PLZF− (filled circles) CD3−CD56dim NK cells from healthy adult CMV-seropositive donors (n = 16) with sizeable pools of adaptive NK cells. (B) The graphs depict perforin, granzyme A and B expression in CD3−CD56dim NK cells from healthy CMV-seronegative (n = 6) and -seropositive donors (n = 6), as well as patients (n = 8) or asymptomatic carriers (n = 2) with GATA2 mutation. (C) The graph depicts expression of T-bet and Eomes in conventional PLZF+ (open circles) and adaptive PLZF− (filled circles) CD3−CD56dim NK cells from healthy adult CMV-seropositive donors with sizeable pools of adaptive NK cells (n = 16). (D) The graphs depict T-bet and Eomes expression in CD3−CD56dim NK cells from healthy CMV-seronegative (n = 6) and -seropositive donors (n = 6), as well as patients (n = 6) or asymptomatic carriers (n = 2) with GATA2 mutation. Mean is indicated, with error bars representing SD. P values were determined using the Student (A,C) paired and (B,D) unpaired t test. **P < .01 and ***P < .001.
Cytotoxic granule constituent and T-bet expression pattern resembling adaptive NK cells in patients with GATA2 mutation. PBMCs, healthy donors, and individuals with heterozygous GATA2 mutations were examined for expression of cytotoxic granule constituents perforin, granzyme A (GzmA), and granzyme B (GzmB) as well as the transcription factors T-bet and Eomes by intracellular flow cytometry. Expression is shown as median fluorescence intensity relative to (R-MFI) CD3+CD8+CD57+ T cells in the same sample. (A) The graph depicts expression of perforin, granzyme A and B in conventional PLZF+ (open circles) and adaptive PLZF− (filled circles) CD3−CD56dim NK cells from healthy adult CMV-seropositive donors (n = 16) with sizeable pools of adaptive NK cells. (B) The graphs depict perforin, granzyme A and B expression in CD3−CD56dim NK cells from healthy CMV-seronegative (n = 6) and -seropositive donors (n = 6), as well as patients (n = 8) or asymptomatic carriers (n = 2) with GATA2 mutation. (C) The graph depicts expression of T-bet and Eomes in conventional PLZF+ (open circles) and adaptive PLZF− (filled circles) CD3−CD56dim NK cells from healthy adult CMV-seropositive donors with sizeable pools of adaptive NK cells (n = 16). (D) The graphs depict T-bet and Eomes expression in CD3−CD56dim NK cells from healthy CMV-seronegative (n = 6) and -seropositive donors (n = 6), as well as patients (n = 6) or asymptomatic carriers (n = 2) with GATA2 mutation. Mean is indicated, with error bars representing SD. P values were determined using the Student (A,C) paired and (B,D) unpaired t test. **P < .01 and ***P < .001.
The expression of cytotoxic granule constituents is controlled, among others, by the T-box transcription factors T-bet and Eomesodermin (Eomes).34 T-bet and Eomes expression in healthy CMV-seropositive donors with sizeable pools of adaptive NK cells as well as in individuals with GATA2 mutation was examined by flow cytometry. Expressed in all NK-cell subsets (supplemental Figure 2A), levels of T-bet were significantly lower in adaptive CD3−CD56dimPLZFlow/− NK cells compared with canonical CD3−CD56dimPLZF+ NK cells, approaching those of CD3+CD8+CD57+ CTL (Figure 3C). Eomes expression displayed a greater variance than T-bet, with no significant differences between NK-cell subsets (Figure 3C). Expression of T-bet was reduced in CD3−CD56dim NK cells from patients with GATA2 mutation, but not asymptomatic carriers, whereas Eomes levels did not significantly differ (Figure 3D). Overall, expression of T-bet and Eomes was positively correlated with expression of perforin and granzyme A, whereas expression of T-bet was negatively correlated with expression of granzyme B (Figure 3B,D; supplemental Figure 2B-C). Thus, human adaptive NK cells display an expression pattern of granule constituents reminiscent of CTL, corresponding to decreased expression of T-bet relative to canonical CD3−CD56dim NK cells. In agreement with the dramatically increased frequencies of NK cells with adaptive features in patients with GATA2 mutation, expression of perforin and T-bet was reduced in the CD3−CD56dim NK cells from these patients.
Residual NK cells from patients with GATA2 mutations display functional characteristics of adaptive NK cells
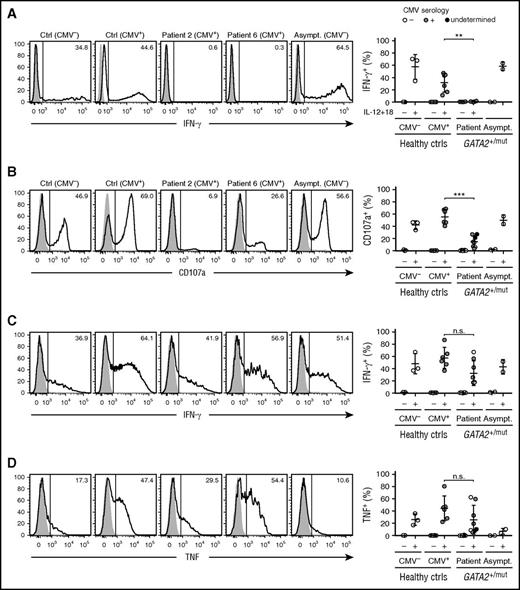
Although adaptive NK cells diversify with respect to surface and intracellular signaling protein expression, they display specific functional attributes.26 Adaptive NK cells do not kill activated autologous T cells or produce IFN-γ in response to innate cytokines IL-12 and IL-18.24 To determine whether NK cells in patients with GATA2 mutation also functionally resembled CMV-associated adaptive NK cells in healthy donors, PBMCs from healthy controls and individuals carrying GATA2 mutation were stimulated with a combination of IL-12 and IL-18. Although NK cells from healthy controls as well as asymptomatic carriers of GATA2 mutation produced IFN-γ, cells from GATA2 patients did not (Figure 4A). Generally, the frequency of CD3−CD56dim NK cells expressing PLZF correlated with the frequency of IFN-γ–producing cells (supplemental Figure 3A). Furthermore, patients with GATA2 mutation retained PLZF-expressing innate mucosal-associated invariant T (MAIT) cells that produced IFN-γ following IL-12 and IL-18 stimulation (supplemental Figure 3B), demonstrating a selective defect in the NK-cell compartment of patients with GATA2 mutation.
Functional properties resembling those of adaptive NK cells in patients with GATA2 mutation. PBMCs from healthy donors, CMV seronegative (n = 3) or seropositive selected for individuals with sizeable expansions of adaptive NK cells (n = 6), as well as patients (n = 4 or n = 8) and asymptomatic carriers (n = 2; asymptomatic carriers 1 and 2) with heterozygous GATA2 mutations were rested overnight, stimulated, and examined for functional responses by flow cytometry. (A) Rested PBMCs were incubated for 24 hours in cell culture medium only or in medium supplemented with IL-12 (10 ng/mL) and IL-18 (100 ng/mL). Histograms show intracellular IFN-γ expression after culture in medium only (filled histograms) or IL-12 and IL-18 (open histograms). Graphs depict frequencies of CD3−CD56dim NK cells expressing IFN-γ. Data from patients 2, 4, 6, and 8 are depicted. (B-D) PBMCs were cocultured with P815 target cells in the presence of 2 μg/mL isotype control IgG (cIgG; filled histograms) or anti-human CD16 (CD16; open histogram) monoclonal antibodies for 6 hours followed by flow cytometric analysis. Histograms show expression of (B) surface CD107a as well as intracellular (C) IFN-γ or (D) TNF in gated CD3−CD56dim NK cells. Graphs depict frequencies of CD3−CD56dim NK cells expressing the indicated functional marker. Data from patients 1, 2, 3, 4, 6, 7, 8, and 9 are depicted.
Functional properties resembling those of adaptive NK cells in patients with GATA2 mutation. PBMCs from healthy donors, CMV seronegative (n = 3) or seropositive selected for individuals with sizeable expansions of adaptive NK cells (n = 6), as well as patients (n = 4 or n = 8) and asymptomatic carriers (n = 2; asymptomatic carriers 1 and 2) with heterozygous GATA2 mutations were rested overnight, stimulated, and examined for functional responses by flow cytometry. (A) Rested PBMCs were incubated for 24 hours in cell culture medium only or in medium supplemented with IL-12 (10 ng/mL) and IL-18 (100 ng/mL). Histograms show intracellular IFN-γ expression after culture in medium only (filled histograms) or IL-12 and IL-18 (open histograms). Graphs depict frequencies of CD3−CD56dim NK cells expressing IFN-γ. Data from patients 2, 4, 6, and 8 are depicted. (B-D) PBMCs were cocultured with P815 target cells in the presence of 2 μg/mL isotype control IgG (cIgG; filled histograms) or anti-human CD16 (CD16; open histogram) monoclonal antibodies for 6 hours followed by flow cytometric analysis. Histograms show expression of (B) surface CD107a as well as intracellular (C) IFN-γ or (D) TNF in gated CD3−CD56dim NK cells. Graphs depict frequencies of CD3−CD56dim NK cells expressing the indicated functional marker. Data from patients 1, 2, 3, 4, 6, 7, 8, and 9 are depicted.
Adaptive NK cells maintain Fc receptor expression and respond to antibody-coated target cells. With respect to responses triggered by Fc receptor engagement, NK cells from patients with GATA2 mutation retained the capacity to degranulate and produce IFN-γ and tumor necrosis factor (TNF) (Figure 4B-D). Together, the data demonstrate that PLZF-deficient NK cells persisting in patients with GATA2 mutation display functional attributes associated with adaptive NK cells in healthy individuals.
GATA-2 is expressed in HSPCs but not in NK-cell progenitors or differentiated cells
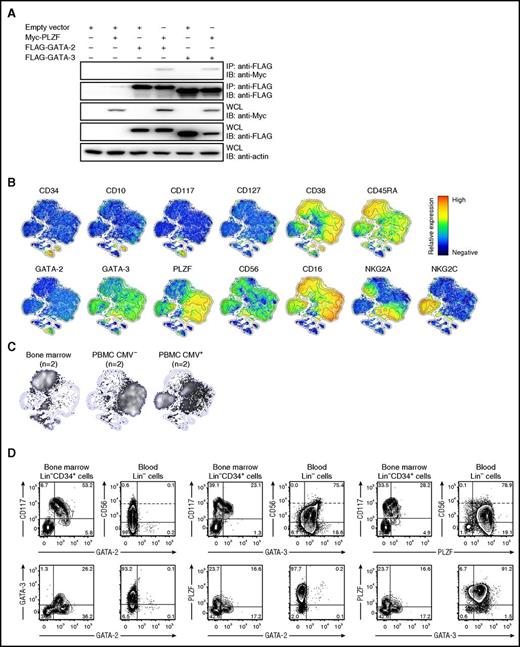
GATA-2 is required for survival and proliferation of HSPCs35 and, interestingly, has been reported to bind PLZF.36 We confirmed this biochemical interaction by transient transfection of 293T cells with constructs encoding tagged transcription factors (Figure 5A). GATA-2 immunoprecipitation resulted in coimmunoprecipitation of PLZF. Similarly, immunoprecipitation of GATA-3, which is expressed in human peripheral blood NK cells,37 also resulted in coimmunoprecipitation of PLZF. Given that western blots suggest weak expression of GATA-2 in CD3−CD56bright NK cells,16 PLZF and GATA-2 could potentially regulate NK-cell differentiation cooperatively. We performed intracellular stainings for PLZF, GATA-2 as well as GATA-3 along with staining for the CD34+ HSPC, lineage, and NK-cell differentiation markers on adult bone marrow and peripheral blood from healthy individuals (Figure 5B-D). As multiparameter stochastic neighbor embedding (SNE) and 2-parameter plots reveal, GATA-2 was expressed in CD34+ HSPCs in blood and bone marrow, but not in CD3−CD56bright or CD3−CD56dim NK cells found in any compartment (Figure 5C-D). Early CD34+ NK-cell progenitors have been associated with acquisition of CD10 and CD45RA expression, as well as increased CD38 expression.38,39 Although GATA-2 expression correlated with that of PLZF, expression of CD10, CD45RA, and CD38 was associated with an absence of GATA-2 expression (Figure 5B), indicating that GATA-2 is expressed in CD34+ HSPCs, but not in committed NK-cell progenitors. In contrast, GATA-3 was uniformly expressed in peripheral blood NK cells with higher expression in CD56bright compared with CD56dim NK cells (Figure 5D), and may therefore constitute an interaction partner of PLZF in mature NK cells.
GATA-2 expression is confined to HSPCs. (A) HEK-293T cells were transfected with empty vector (EV), or constructs encoding FLAG-tagged GATA-2 or GATA-3 and Myc-tagged PLZF. Twenty-four hours after transfection, GATA-2 or GATA3 were immunoprecipitated and precipitates as well as whole-cell lysates (WCL) were analyzed by western blotting with antibodies, as indicated. (B-D) Bone marrow, cord blood, or PBMCs from healthy volunteers were surface stained with antibodies to lineage and differentiation markers as well as intracellular with antibodies to GATA-2, GATA-3, and PLZF. (B) Barnes-Hut t-distributed stochastic neighbor embedding (t-SNE) analysis of 13-parametric data were performed on gated CD3−CD14−CD19−CD123− cells combined from samples of bone marrow (n = 2) and peripheral blood of CMV seronegative (n = 2) and CMV seronegative (n = 2) from healthy volunteers. Plots show expression of surface and intracellular proteins, as indicated, in the 2-dimensional t-SNE field with relative expression denoted as red when high and blue when absent. (C) Cell density in the t-SNE field for pooled samples from bone marrow (n = 2) and peripheral blood of CMV-seronegative (n = 2) and CMV-seropositive (n = 2) donors, respectively. (D) Contour plots show expression of the indicated markers in bone marrow (CD3−CD14−CD19−CD123−) Lin−CD34+ cells as well as peripheral blood Lin− lymphocytes. In plots depicting CD56 expression, dotted lines discriminate CD56bright from CD56dim cells. Plots are from representative donors.
GATA-2 expression is confined to HSPCs. (A) HEK-293T cells were transfected with empty vector (EV), or constructs encoding FLAG-tagged GATA-2 or GATA-3 and Myc-tagged PLZF. Twenty-four hours after transfection, GATA-2 or GATA3 were immunoprecipitated and precipitates as well as whole-cell lysates (WCL) were analyzed by western blotting with antibodies, as indicated. (B-D) Bone marrow, cord blood, or PBMCs from healthy volunteers were surface stained with antibodies to lineage and differentiation markers as well as intracellular with antibodies to GATA-2, GATA-3, and PLZF. (B) Barnes-Hut t-distributed stochastic neighbor embedding (t-SNE) analysis of 13-parametric data were performed on gated CD3−CD14−CD19−CD123− cells combined from samples of bone marrow (n = 2) and peripheral blood of CMV seronegative (n = 2) and CMV seronegative (n = 2) from healthy volunteers. Plots show expression of surface and intracellular proteins, as indicated, in the 2-dimensional t-SNE field with relative expression denoted as red when high and blue when absent. (C) Cell density in the t-SNE field for pooled samples from bone marrow (n = 2) and peripheral blood of CMV-seronegative (n = 2) and CMV-seropositive (n = 2) donors, respectively. (D) Contour plots show expression of the indicated markers in bone marrow (CD3−CD14−CD19−CD123−) Lin−CD34+ cells as well as peripheral blood Lin− lymphocytes. In plots depicting CD56 expression, dotted lines discriminate CD56bright from CD56dim cells. Plots are from representative donors.
Impaired NK-cell differentiation from lineage-negative cells in individuals with GATA2 mutation
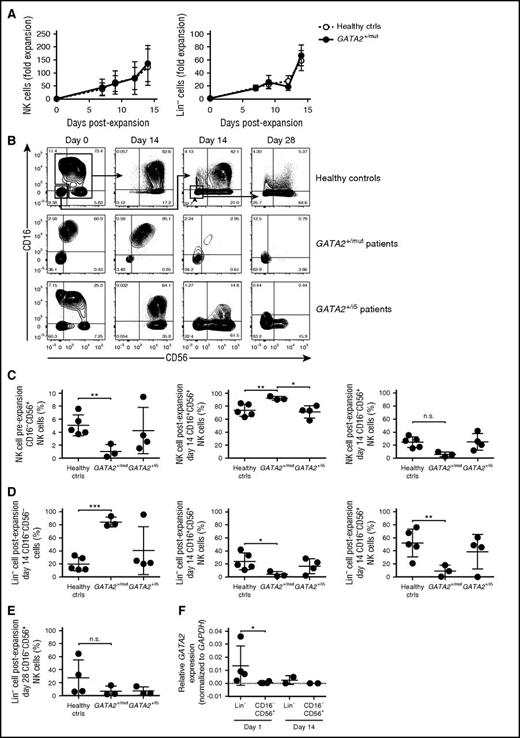
To further understand the impact of GATA-2 haploinsufficiency on NK-cell differentiation and proliferation, we adopted an ex vivo NK-cell expansion method with the potential to expand NK-cell progenitors from the blood.40,41 Blood samples from healthy controls and from 7 individuals with heterozygous GATA2 mutation were studied (supplemental Table 3). Sorted total NK (CD3−CD20−CD14− and CD16+ and/or CD56+) cells or lineage-negative (Lin−; CD3−CD20−CD14−CD16−CD56−) cells were cultured for 14 days at which time phenotyping and resorting was performed. The Lin− cell population contains circulating CD34+ HSPCs which encompass stage 1 and 2 NK-cell progenitors, as well as low frequencies of CD34−CD117+CD161+CD94− stage 3 and CD94+ stage 4–like NK-cell progenitors (supplemental Figure 4).38 Freshly sorted total NK cells and Lin− cells from patients and healthy controls showed comparable 14-day overall expansion kinetics in the presence of B-LCL feeder cells and IL-2 (Figure 6A), suggesting that GATA-2 haploinsufficiency did not markedly impair cell proliferation. However, there were differences in the type of cells produced. Total NK cells from controls produced primarily CD16+CD56+ and a smaller population of CD16−CD56+ after 14 days of expansion culture. In contrast, total NK cells expanded from GATA2+/mut patients (mutation affecting coding sequence or exon-intron splice sites) produced significantly reduced numbers of CD16−CD56+ cells, whereas expansion of total NK cells from GATA2+/i5 patients (intronic mutation in intron 5) resulted in more variable proportions of CD16−CD56+ cells after expansion (Figure 6B-C). Sorted Lin− cells from healthy controls generated a significant CD56+ (either CD16dimCD56+ or CD16−CD56+) cell population at day 14 (Figure 6B,D), indicating the presence of progenitors for CD56+ NK cells within the Lin− cell population. Re-sorting and restimulation of control Lin− cells after the first 2 weeks of culture consistently generated more CD56+ NK cells at day 28 (Figure 6B,E). In contrast, Lin− cells from GATA2+/mut patients failed to generate CD56+ NK cells at day 14 or day 28 (Figure 6B,E), consistent with a diminished frequency of Lin−CD34−CD117+CD161+CD94− NK-cell progenitors (supplemental Figure 4C). Lin− cells from GATA2+/i5 patients generated variable numbers of CD56+ NK cells at day 14, with a trend toward fewer CD16−CD56+ cells at day 28 (Figure 6B,D). Finally, corroborating our flow cytometry stainings, GATA2 transcription was detected in Lin− cells, but not in CD3−CD16−CD56+ NK cells (Figure 6F). Our findings indicate that the NK-cell progenitor pool is markedly diminished in individuals with GATA2 mutation.
Paucity of NK-cell progenitors in blood from patients with GATA2 mutation. NK-cell or CD3−CD20−CD14−CD16−CD56− (Lin−) cell populations were cultured with irradiated B-LCL feeder cells and IL-2. (A) Charts depict expansion kinetics of total NK cells and Lin− cells up to 14 day of ex vivo expansion. (B) Representative flow plots of control, GATA2 exonic/splicing mutation, and GATA2 intron 5 mutation. CD16 and CD56 expression on gated mononuclear CD3−CD20−CD14− cells is shown. Total NK cells and Lin− cells were sorted and cultured separately for 14 days. Lin− cells were sorted and stimulated again from day 14 Lin− cell culture for another 14 days (shown as day 28). (C-E) NK-cell subpopulations frequencies of (C) pre-expansion, day 14 total NK cells, (D) day 14 Lin− cells, and (E) day 28 Lin− cells are shown in comparison. (F) Relative GATA2 expression levels in sorted control Lin− cells compared with control CD16−CD56+ cells at day 1 (n = 4) and day 14 (n = 2) ex vivo culture. Bars indicate mean ± SD. *P < .05, **P < .01, and ***P < .01.
Paucity of NK-cell progenitors in blood from patients with GATA2 mutation. NK-cell or CD3−CD20−CD14−CD16−CD56− (Lin−) cell populations were cultured with irradiated B-LCL feeder cells and IL-2. (A) Charts depict expansion kinetics of total NK cells and Lin− cells up to 14 day of ex vivo expansion. (B) Representative flow plots of control, GATA2 exonic/splicing mutation, and GATA2 intron 5 mutation. CD16 and CD56 expression on gated mononuclear CD3−CD20−CD14− cells is shown. Total NK cells and Lin− cells were sorted and cultured separately for 14 days. Lin− cells were sorted and stimulated again from day 14 Lin− cell culture for another 14 days (shown as day 28). (C-E) NK-cell subpopulations frequencies of (C) pre-expansion, day 14 total NK cells, (D) day 14 Lin− cells, and (E) day 28 Lin− cells are shown in comparison. (F) Relative GATA2 expression levels in sorted control Lin− cells compared with control CD16−CD56+ cells at day 1 (n = 4) and day 14 (n = 2) ex vivo culture. Bars indicate mean ± SD. *P < .05, **P < .01, and ***P < .01.
Discussion
Haploinsufficiency of the transcription factor GATA-2 causes HSPC attrition, leading to deficiencies in monocytes, DCs, B cells, and NK cells and precipitating clinical symptoms of immunodeficiency, lymphedema, as well as MDS.1,2 An estimated 20% of individuals with GATA2 mutation and lymphopenias retain NK cells.1,16 We determined that GATA-2 is expressed in HSPCs, but not in committed NK-cell progenitors. Rather, CD56bright and CD56dim NK cells expressed GATA-3. A previous study reported a paucity of B-/NK-cell progenitors in patients with GATA2 mutation,14 and our culture system failed to generate NK cells from Lin− progenitor cells in such patients. Performing a variety of phenotypical and functional assays, we found that NK cells in such patients overwhelmingly displayed features of adaptive NK cells.26,28 Specifically, they lacked expression of PLZF, displayed distinct, clonal-like yet variable expression patterns of surface receptors and signaling proteins, expressed levels of cytotoxic granule contents associated with adaptive NK cells and CTLs, and did not produce IFN-γ in response to IL-12 and IL-18. Such extreme biases in the NK-cell population toward adaptive phenotypes have not been observed in healthy individuals.24 Remarkably, such differentiated NK cells persisted in the apparent absence of progenitors, akin to CMV-induced adaptive NK cells in mice.23
The molecular mechanisms of adaptive NK-cell differentiation and homeostasis are poorly defined.28 The fact that adaptive NK cells comprised the total residual NK-cell population in symptomatic patients with heterozygous GATA2 mutation could be the result of either sustained output of adaptive NK cells due to developmental pathways less dependent on GATA-2 or, following depletion or dysfunction of the myeloid and lymphoid progenitor cell pool, peripheral survival, and proliferation of adaptive NK cells. GATA-2 binds a promoter element of Nfil3, encoding a transcription factor that promotes cell survival.42-44 Nfil3-deficient mice display a severe defect in NK-cell development,45,46 in addition to impaired development of CD8+ and CD103+ conventional DC subsets, B cells, and other innate lymphoid subsets.44,47-49 Reduced GATA-2–mediated induction of NFIL-3 could thus contribute to DC, B-cell, and NK-cell cytopenias observed in humans with GATA-2 haploinsufficiency, as suggested by our findings of reduced NK-cell output from Lin− cell progenitors in patients with GATA2 mutation. Interestingly, CMV infection bypasses the requirement for Nfil3 in mouse NK-cell development, supporting generation of long-lived, adaptive NK cells.50 Moreover, in mice, Nfil3 is required for the development of Eomes-expressing NK cells, but not liver or salivary gland NK cells that are TRAIL+Eomes−.51-53 Conceivably, GATA-2 haploinsufficiency in humans could thus bias for GATA-2–independent differentiation and expansion of adaptive NK cells. However, patients with GATA2 mutation generally present with severe infections, yet a majority lack sizeable peripheral numbers of inflammation-driven adaptive NK cells,1,16 similar to most healthy adults.24 We thus interpret our data as supporting a requirement for GATA-2–expressing HSPCs and canonical CD56dim NK-cell progenitors for initial development of adaptive NK cells (supplemental Figure 5). Once hematopoietic progenitor deficiencies develop in patients with GATA2 mutation, adaptive NK-cell differentiation may also be limited by reduced numbers of accessory immune cells. In settings of HSPC transplantation, monocytes are required for adaptive NK-cell differentiation.54
Deutrium-labeling experiments in healthy adults have indicated a 14-day half-life of blood NK cells.55 Prolonged survival and self-renewal could explain the vast overrepresentation of adaptive NK cells observed in patients with GATA2 mutation. Supporting this notion, human adaptive NK cells express higher levels of antiapoptotic Bcl-2, with epigenetically unique populations persisting for at least 35 months.24,56 PLZF has been demonstrated to confer a proapoptotic phenotype to invariant natural killer T cells (iNKT) and MAIT cells.57 Accordingly, iNKT precursors in Itk-deficient mice with elevated levels of PLZF, EOMES, CD122 display increased rates of apoptosis.58 Reduced expression of PLZF could thus support adaptive NK-cell longevity, explaining the persistence of adaptive NK cells in symptomatic individuals with GATA2 mutation devoid of canonical NK-cell progenitors. IL-15–dependent self-renewal maintains CD8+ T-cell memory.59,60 Homeostatic proliferation may also contribute to adaptive NK-cell persistence, although only low-level Ki-67 expression is detected in these cells (data not shown). Moreover, in vitro culture experiments suggested a diminished proliferative capacity of NK cells from patients with GATA2 mutation.
Although PLZF interacts with GATA-2,36 our findings argue against GATA-2/PLZF cooperativity in progenitors and differentiated NK cells, as GATA-2 expression was confined to HSPCs. CMV establishes lifelong latency in CD34+ cells and myeloid progenitors, and expression of CMV latency-associated genes is, at least partially, GATA-2 dependent.61 Latency is maintained by suppression of miR-92a leading to upregulation of GATA-2, promoting IL-10 production that inhibits T-cell immunity and stem cell apoptosis.61 Hypothetically, CMV-infected individuals with heterozygous GATA2 mutation may have impaired IL-10 induction, contributing to an inflammatory milieu driving stem cell exhaustion. Infections in general and CMV in particular could thus play a role in the pathology of GATA2 haploinsufficiency.
With respect to disease, it is not clear whether adaptive NK cells may protect, for example, by counteracting viral infections, or promote progression. Emergence of CMV-associated adaptive NK cells may protect from virus reactivation. Through strong Fc receptor CD16-mediated effector responses, adaptive NK cells may even increase antibody-dependent resistance to other intracellular infections.24,25 However, adaptive NK cells are major producers of proinflammatory mediators IFN-γ and TNF, cytokines that have been shown to promote HSPC differentiation and exhaustion during stress or demand-adapted hematopoiesis, even inducing HSPC destruction by upregulating apoptosis-related genes such as FAS and caspases.62,63 In patients, exacerbated levels of these cytokines may result from adaptive NK-cell activation or diminished immunoregulation of activated T cells.24 As such, although beneficial for killing of infected cells, high frequencies of adaptive NK cells in individuals with GATA2 mutation could contribute to progressive, inflammation-driven bone marrow failure. Notably, in our limited material, asymptomatic carriers with GATA2 mutation were mostly CMV seronegative and retained normal numbers of conventional NK cells whereas several patients had increased frequencies of CD56− NK cells, associated with chronic viral infections.
In summary, we find that GATA-2 haploinsufficiency is associated with attrition of NK-cell progenitors, abolishing canonical NK-cell differentiation. Nonetheless, adaptive NK cells may persist in patients with GATA2 mutation, indicating that they are considerably more long-lived than canonical NK cells. Further support for longevity and self-renewal of adaptive NK cells comes from studies of paroxysmal nocturnal hemoglobinuria patients, where Corat and colleagues document that glycosylphosphatidylinositol-expressing adaptive NK cells persist in spite of glycosylphosphatidylinositol-deficient HSPCs progressively dominating the bone marrow.64 These findings provide insights into a mechanism that may ensure durable antiviral immunity,28 with potential implications for strategies aimed at providing lasting NK-cell–based immunotherapy of cancer.65,66
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Philip J. McCoy and Ann Williams of the Flow Cytometry Core Facility and Keyvan Keyvanfar from Hematology Branch, National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) for flow cytometry support.
This work was supported by the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC grant agreement no. 311335, Swedish Research Council, Norwegian Research Council, Swedish Foundation for Strategic Research, Wallenberg Foundation, Swedish Cancer Foundation, Swedish Childhood Cancer Foundation, as well as the Stockholm County Council and Karolinska Institutet Center for Innovative Medicine (Y.T.B.), as well as the intramural program of the NHLBI (C.E.D.).
Authorship
Contribution: H.S. designed experiments, performed phenotypical and functional experiments on healthy volunteers and individuals with GATA2 mutation, analyzed data, and wrote the manuscript; M.J. designed experiments, sorted and expanded cells from healthy volunteers and individuals with GATA2 mutation, analyzed data, and contributed to the writing of the manuscript; H.H. designed experiments, performed transcription factor interaction studies, and analyzed data; J.T. performed multiparametric flow cytometry data analyses; S.C.C.C. and T.D.H. optimized experiments and analyzed data; D.S.J.A. analyzed the Lin− cell population; W.W. and K.R.C. performed FLT-3L assays; V.B., J.K.D.-M., R.E.D., A.P.H., D.T., T.W., P.A., I.N., and S.M.H. took care of patients, collected clinical data, and provided samples; M.C. provided clinical material, analyzed data, and commented on the manuscript; C.E.D. designed experiments, analyzed data, and contributed to the writing of the manuscript; and Y.T.B. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yenan T. Bryceson, Center for Hematology and Regenerative Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, Hälsovägen 7, S-141 57 Stockholm, Sweden; e-mail: yenan.bryceson@ki.se.
References
Author notes
H.S. and M.J. have contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal