In this issue of Blood, Fulciniti et al report on the role of p21-activated kinase 4 (PAK4), a member of the p21-activated kinases family, in myeloma cell survival and proliferation. They also describe the effects of a novel allosteric inhibitor of PAK4 that is currently in early phase 1 trials.1
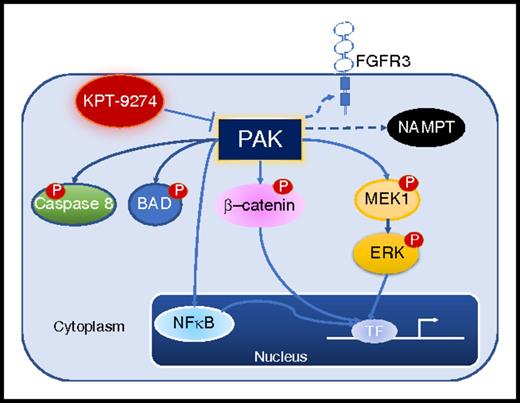
Selected PAK substrates in transformed cells. Dashed arrows indicate novel PAK4 substrates (FGFR3 signaling and NAMPT) reported by Fulciniti et al in myeloma cell lines. P, phosphoryl-; TF, transcription factor.
Selected PAK substrates in transformed cells. Dashed arrows indicate novel PAK4 substrates (FGFR3 signaling and NAMPT) reported by Fulciniti et al in myeloma cell lines. P, phosphoryl-; TF, transcription factor.
The 6 hallmarks of cancer highlight the quintessential role kinases play in the signal transduction across complex and deregulated networks of circuits required for cancer cell survival, proliferation, motility, and invasion.2 Therefore, pharmacologic drugging of these kinases represents an attractive and logical target, but not without 2 major challenges. The first stems from the kinases' ubiquitous tissue distribution among transformed and normal cells, and the second from achieving kinase selectivity due to the evolutionary conservation of the adenosine triphosphate (ATP)–binding pocket among kinases.
The PAKs were first discovered in 1994 with the identification of PAK1 in a screen for proteins that interact with the small G-proteins, Rac1 and Cdc42.3 Currently, the PAKs family consists of 6 mammalian serine/threonine kinases grouped into 2 subfamilies, based on their domains' structural homology and regulatory mechanisms. Group I PAKs (PAK1-3) are activated upon binding the ρ-family of GTPases, Cdc42 and Rac1, whereas group II PAKs (PAK4-6) are constitutively phosphorylated and active, independent of GTPases.4 PAKs group I and II have been implicated in the pathogenesis of several malignancies.5 In the particular case of the PAKs family member PAK4, it was initially recognized as a key mediator of Ras signaling, Ras-mediated cellular transformation, and in vivo tumorigenesis. In addition, PAK4 represents an essential node in cancer cells' circuits regulating proliferation (G1 phase cell cycle progression and mitotic spindle formation), apoptotic cell death effectors (BCL2-associated agonist of cell death [BAD] phosphorylation and caspase 8 cleavage), and the transduction of survival signals activating the nuclear factor (NF)–κB, MEK-extracellular signal-regulated kinase (ERK), and WNT/β-catenin pathways.6,7 Lastly, it is important to note that PAK4 genomic locus (19q13.2) is frequently amplified in several tumors, including multiple myeloma (MM).
In light of the strong evidence implicating PAK4 in cancer pathogenesis, it is not surprising that a number of PAK family inhibitors have been developed, of which only one, the ATP-competitive PAK4 inhibitor (PF-3758309; Pfizer), was clinically tested in a phase 1 trial. However, the development of this compound was prematurely halted due to undesirable pharmacokinetics (low human bioavailability) and lack of a dose-response effect despite very promising preclinical activity.8 It is important to note here that the phenotype of PAK4-null mice, which is embryonically lethal, involves fetal heart defects as well as abnormal neuronal development. Therefore, the clinical safety and feasibility of such an approach targeting PAK4 is yet to be clinically demonstrated, and hence the relevance of the work reported in this issue of Blood by Fulciniti et al describing the effects of a novel allosteric PAK4 inhibitor KPT-9274 in MM.
Similar to the role of PAK4 in other malignancies, PAK4 is here reported to be expressed in most myeloma cell lines and primary myeloma cells, and it is demonstrated to regulate myeloma cells proliferation and survival through activation of the NF-κB and MEK-ERK canonical pathways. The authors also describe, in a series of quantitative proteomics and coimmunoprecipitation experiments, the selective affinity of KPT-7523 (Karyopharm) for the PAKs group II family member PAK4. Consistent with its allosteric inhibitory effects, KPT-7523 is shown to bind the C-terminal kinase but not the N-terminal regulatory domain of PAK4. Furthermore, and based on improved absorption, distribution, metabolism, and excretion/pharmacokinetic (ADME/PK) properties, a KPT-7523 analog, KPT-9274, is shown to be highly cytotoxic to myeloma cell lines and primary myeloma cells (50% inhibitory concentration [IC50] in the nanomolar range), with an even enhanced toxicity in cells harboring a t(4;14) or FGFR3 gene mutation. Of interest, the authors identified a direct binding between PAK4 and FGFR3, suggesting that PAK4 may be directly interacting with FGFR3. However, the authors did not provide any evidence or mechanistic explanation of how this interaction regulated FGFR3 signaling, in particular in cell lines such as OPM2 harboring a K650E mutation that results in the strong and constitutive activation of the FGFR3 tyrosine kinase. Lastly, the authors also provide evidence that PAK4 formed a stable complex with nicotinamide phosphoribosyltransferase (NAMPT), a key regulator of the intracellular nicotinamide adenine dinucleotide. This interaction was also disrupted by KPT-9274 and hence may represent another mechanism through which PAK4 inhibition may affect myeloma cells survival (see figure).
Is there enough reason to believe that KPT-7532 and its analogs will succeed where other PAK family inhibitors thus far fell short? A couple of features may indeed support this statement. First, the selectivity for PAK4 targeting coupled with its high affinity and low cytotoxic IC50s are surely in favor of KPT-9274. Indeed, its allosteric inhibitory properties (not competing for the evolutionarily conserved ATP-binding pocket) overcome the selectivity challenge that often hinders the therapeutic development of kinase inhibitors and may also minimize the risk of acquired clinical resistance that usually results from acquired mutations in the kinase ATP-binding domain. In addition, the targeting of oncogenic FGFR3 signaling and its destabilizing interaction with NAMPT may offer additional therapeutic benefits. On the other hand, and because the prosurvival effects of PAK4 in myeloma cells appear to be largely mediated through MEK-ERK signaling, it is reasonable to argue that PAK4 inhibition may not offer any benefit beyond that of a direct MEK inhibitor (already in the clinic). Furthermore, PAK4 is ubiquitously expressed in normal tissues, raising some concerns regarding the potential toxicities that may be associated with the targeting of this kinase. Surely, “the proof of the pudding is in the eating.” A phase 1 trial of KPT-9274 is currently underway and will examine the clinical safety and feasibility of PAK4 inhibition (#NCT02702492, www.clinicaltrials.gov). Nevertheless, and while awaiting the results of this ongoing phase 1 trial, Fulciniti et al provided us in the meantime with convincing evidence that PAK4 represents a legitimate target in myeloma cells, and perhaps it is about time for myeloma cells to start PAKing!
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal