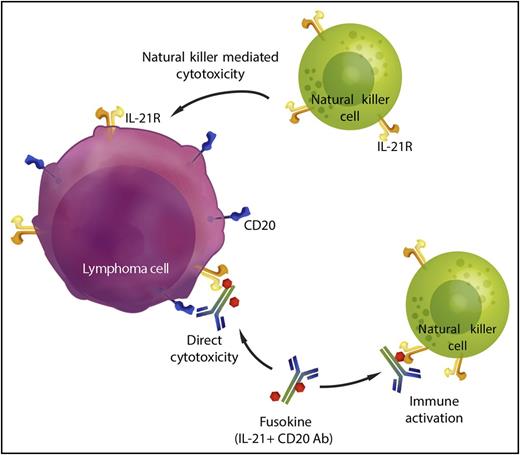
In this issue of Blood, Bhatt et al have shown that fusing interleukin 21 (IL-21) to an anti-CD20 antibody results in a molecule that has superior antilymphoma activity than each of its individual components.1 They find that the fused IL-21 induces direct cytotoxicity to the lymphoma cells but also activates immune effector cells that enhance the efficacy of the anti-CD20 antibody. The addition of IL-21 to an anti-CD20 antibody to form an anti-CD20-IL-21 fusokine therefore provides enhanced activity in 2 ways: firstly, it increases cytotoxicity directly targeting the malignant cell, and secondly, it modulates and augments the anti-tumor immune response (see figure).
Anti-CD20-IL-21 fusokine. IL-21 conjugated to an anti-CD20 antibody provides 2 additional mechanisms of cytotoxicity. The fusokine binds to CD20+ lymphoma cells and IL-21 signaling has a direct cytotoxic effect on the lymphoma cells. IL-21 also activates intratumoral natural killer (NK) cells and promotes lymphoma-directed cytotoxicity by effector cells. Professional illustration by Somersault18:24.
Anti-CD20-IL-21 fusokine. IL-21 conjugated to an anti-CD20 antibody provides 2 additional mechanisms of cytotoxicity. The fusokine binds to CD20+ lymphoma cells and IL-21 signaling has a direct cytotoxic effect on the lymphoma cells. IL-21 also activates intratumoral natural killer (NK) cells and promotes lymphoma-directed cytotoxicity by effector cells. Professional illustration by Somersault18:24.
Established anti-CD20 antibody treatments such as rituximab have made a profound impact on the treatment of B-cell non-Hodgkin lymphoma (NHL).2 In multiple studies in both indolent and aggressive B-cell NHL, the addition of rituximab has resulted in increased response rates, prolonged progression-free survival, and in many cases, improved overall survival. Although various approaches have been attempted to improve CD20 antibody efficacy,3 fusing a cytokine to the antibody is a unique approach and profoundly enhances its benefit. The presence of IL-21 in close proximity to rituximab clearly is significantly beneficial and may be due to activating immune cells in close proximity to the malignant clone.4
In the past, IL-21 has been administered systemically in combination with rituximab.5 This resulted in generalized, systemic immune activation rather than specific activation at the site of lymphoma. In a phase 1 clinical trial, IL-21 was IV administered, but did result in toxicities including nausea, vomiting, diarrhea, hypotension, and edema. Although clinical responses were seen, even in rituximab refractory patients, and the durability of response to the combination appeared longer than the previous response to rituximab-based therapy, the systemic administration of IL-21 did not appear to significantly enhance the efficacy of rituximab.
In the study by Bhatt et al, they found that fusing IL-21 to rituximab to generate a fusokine molecule led to direct apoptosis of lymphoma cells, including those that were resistant to IL-21 treatment alone. Furthermore, they found that the fusokine enhanced NK cell activation, resulting in increased cytokine production by effector cells and greater antibody-dependent cytotoxicity. These findings strongly suggest that local action of IL-21 rather than systemic administration may clearly provide a significant therapeutic advantage.6
Unique molecules such as the anti-CD20-IL-21 fusokine molecule provide a proof of principle that local delivery of a cytokine such as IL-21 in the context of antibody binding to the malignant cell may have profound therapeutic advantages, and may be better than administering these components systemically. Clearly, additional studies will be necessary to assess whether the agent is safe and effective, and clinical trials are planned. However, these early preclinical studies suggest that a fusokine molecule such as this may be highly effective in treating lymphoma. The addition of IL-21 to an anti-CD20 antibody in this fashion is truly a case of “the tail wagging the dog.”
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal