The SAMD9L gene and its paralog SAMD9, sitting head to tail on chromosome 7q, are among the notable absences in −7/7q− myelodysplastic syndromes (MDSs). As with many other genes harboring somatic mutation in neoplasia, germ line variants often provide critical insights into the mechanisms of dysfunction. In this issue of Blood, Tesi et al provide tantalizing new details to the story of SAMD9L mutation and familial −7/7q− syndromes.1 This widely expressed protein normally functions to inhibit proliferation and is therefore a potential tumor suppressor gene. The authors find 2 novel gain-of-function (GoF) variants that are associated with cytopenias, immunodeficiency, and neurological dysfunction and show how these can be ameliorated by coinherited loss-of-function alleles, or by somatic reversion of the mutated alleles in the bone marrow. When the progenitor ecosystem fails to select benign revertant clones, a predisposition to −7/7q− MDS is observed, otherwise known as the familial condition of ataxia-pancytopenia syndrome (ATXPC; Mendelian Inheritance in Man no. 159550). Neurological consequences, although variable, are not remedial by reversion mutation.
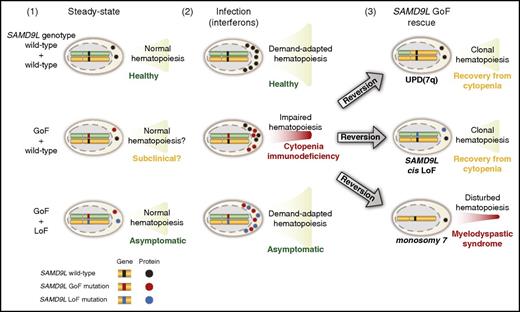
Hypothetical model of the pathophysiology of germ line SAMD9L GoF mutations in relation to hematopoietic stem and progenitor cell proliferation and differentiation. Healthy individuals, with 2 wild-type SAMD9L copies (top row), have (1) normal, steady-state hematopoiesis and (2) increased cellular output upon infection-induced, demand-adapted hematopoiesis. In contrast, carriers of heterozygous SAMD9L GoF mutations (middle row) may (1) display grossly normal (subclinical?) hematopoiesis for some time, but (2) experience cytopenias and immunodeficiency upon infections early in life. In this setting, interferons can promote SAMD9L expression, with SAMD9L GoF mutants acting as potent suppressors of cell proliferation, dramatically impairing hematopoiesis and immunity. The ensuing hematopoietic crisis can facilitate (3) selection and expansion of revertant mutants, by uniparental disomy (UPD) of 7q, SAMD9L loss-of-function (LoF) mutations in cis, or monosomy 7. Whereas UPD(7q) and in cis SAMD9L, LoF mutations can support clonal hematopoiesis and recovery from cytopenia, monosomy 7 is associated with development of myelodysplastic syndrome. Finally, carriers of combined SAMD9L GoF mutation and rare LoF variants in trans (bottom row) are asymptomatic, suggesting they have normal (1) steady-state and (2) demand-adapted hematopoiesis. As such, pathogenic effects of SAMD9L GoF mutations may be balanced by SAMD9L LoF mutations.
Hypothetical model of the pathophysiology of germ line SAMD9L GoF mutations in relation to hematopoietic stem and progenitor cell proliferation and differentiation. Healthy individuals, with 2 wild-type SAMD9L copies (top row), have (1) normal, steady-state hematopoiesis and (2) increased cellular output upon infection-induced, demand-adapted hematopoiesis. In contrast, carriers of heterozygous SAMD9L GoF mutations (middle row) may (1) display grossly normal (subclinical?) hematopoiesis for some time, but (2) experience cytopenias and immunodeficiency upon infections early in life. In this setting, interferons can promote SAMD9L expression, with SAMD9L GoF mutants acting as potent suppressors of cell proliferation, dramatically impairing hematopoiesis and immunity. The ensuing hematopoietic crisis can facilitate (3) selection and expansion of revertant mutants, by uniparental disomy (UPD) of 7q, SAMD9L loss-of-function (LoF) mutations in cis, or monosomy 7. Whereas UPD(7q) and in cis SAMD9L, LoF mutations can support clonal hematopoiesis and recovery from cytopenia, monosomy 7 is associated with development of myelodysplastic syndrome. Finally, carriers of combined SAMD9L GoF mutation and rare LoF variants in trans (bottom row) are asymptomatic, suggesting they have normal (1) steady-state and (2) demand-adapted hematopoiesis. As such, pathogenic effects of SAMD9L GoF mutations may be balanced by SAMD9L LoF mutations.
As the authors observe, heterozygous loss of SAMD9L function is well-tolerated when inherited in the germ line, and recurrent SAMD9L mutation is not observed in sporadic MDS (see figure).2 Although knockout mice are predisposed to develop acute myeloid leukemia, these results imply that additional loss of genes such as EZH2, CUX1, MLL3, or DOCK4 contribute to the 7q− syndrome in humans.3 The curious observation is therefore that SAMD9L mutation does not directly cause malignancy, as might be expected for deleterious mutation of a tumor suppressor gene. Rather, it is the GoF that causes disease by restricting hematopoiesis to the point that pathologically adapted clones emerge, a phenomenon previously described in other tissues as “compensatory aneuploidy.”4 In the case of hematopoiesis, however, the appearance of such −7/7q− clones foreshadows the development of a high-risk malignancy.
Germ line SAMD9L mutation was recently identified in 2 pedigrees of ATXPC, including the index family, by Chen et al.5 These authors speculated that GoF mutation and consequent suppression of cell proliferation might lead to the selection of 7q− clones. Evidence in favor of this mechanism is now presented by Tesi et al through in vitro experiments. In addition, these author provide insightful comments about the potential role of viral infection and interferon production in driving the selection of beneficial revertant or pathological aneuploid clones. Induction of SAMD9L by interferon is proposed as a mechanism of feedback inhibition of emergency hematopoiesis that might explain the physiological transient suppression of hematopoiesis during infection. Carefully documented infections in the patients were followed by profound cytopenia and then remission, suggesting that reversion mutation had occurred at this point. Further nuances were observed in the lineages that harbor revertants. Higher reversion frequency was observed in B and natural killer (NK) cells, suggesting that escape from oversuppression by SAMD9L was more important for maintaining these lineages and indirectly supporting the concept of lineage-primed stem cells.6 The stage is now set to develop GoF models to explore the stress response of progenitors and potential role of SAMD9L in lineage specification.
Although many familial cases of MDS remain without a genetic etiology, SAMD9L joins a growing list of germ line variants that predispose to cytopenia and malignant transformation.7 A differential diagnosis is likely to included severe forms of dyskeratosis congenita, which may also affect the nervous system,8 or heterozygous GATA2 mutation, which may appear first as immunodeficiency, similar to at least 1 SAMD9L pedigree with viral illness, intracellular infections, and pulmonary alveolar proteinosis.9 However, although deafness may occur with GATA2 mutation, advanced neurological deficit would not be expected. The authors also noted that CD56bright immature NK cells were preserved and Fms-like tyrosine kinase-3 ligand was only modestly elevated, in contrast to GATA2 mutation. The high frequency of reversion mutation adds further complexity to the diagnosis of SAMD9L GoF because, at the presentation of MDS, this allele will have been lost from the myeloid lineages in peripheral blood, requiring a sample of nonhematopoietic tissue for genetic screening.
Overall, the studies performed on these new cases of SAMD9L mutation illustrate the exquisite long-term balance in growth regulation that must be maintained within the human progenitor compartment. Much remains to be learned about how SAMD9L and its paralog SAMD9 are involved, including their interactions with each other. Recent evidence points to a role in endosome fusion and recycling of growth factor receptors.10 With apologies to Dr Seuss and his beloved character Sam-I-Am, the final question is of therapeutic potential. We would like to ask SAMD9L: Would you? Could you?
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal