Key Points
Rituximab exposure decreased as metabolic tumor volume increased, and correlated with metabolic response and survival.
Rituximab dose could be individualized according to metabolic tumor volume to achieve optimal exposure and therefore optimal response.
Abstract
High variability in patient outcome after rituximab-based treatment is partly explained by rituximab concentrations, and pharmacokinetic (PK) variability could be influenced by tumor burden. We aimed at quantifying the influence of baseline total metabolic tumor volume (TMTV0) on rituximab PK and of TMTV0 and rituximab exposure on outcome in patients with diffuse large B-cell lymphoma (DLBCL). TMTV0 was measured by 18F-fluorodeoxyglucose-positron emission tomography-computed tomography in 108 previously untreated DLBCL patients who received four 375 mg/m2 rituximab infusions every 2 weeks in combination with chemotherapy in 2 prospective trials. A 2-compartment population model allowed describing rituximab PK and calculating rituximab exposure (area under the concentration-time curve; AUC). The association of TMTV0 and AUC with metabolic response after 4 cycles, as well as progression-free survival (PFS) and overall survival (OS), was assessed using logistic regression and Cox models, respectively. Cutoff values for patient outcome were determined using receiver operating characteristic curve analysis. Exposure to rituximab decreased as TMTV0 increased (R2 = 0.41, P < .0001). A high AUC in cycle 1 (≥9400 mg × h per liter) was associated with better response (odds ratio, 5.56; P = .0006) and longer PFS (hazard ratio [HR], 0.38; P = .011) and OS (HR, 0.17; P = .001). A nomogram for rituximab dose needed to obtain optimal AUC according to TMTV0 was constructed, and the 375 mg/m2 classical dose would be suitable for patients with TMTV0 <281 cm3. In summary, rituximab exposure is influenced by TMTV0 and correlates with response and outcome of DLBCL patients. Dose individualization according to TMTV0 should be evaluated in prospective studies. These studies were registered at www.clinicaltrials.gov as #NCT00498043 and #NCT00841945.
Introduction
Rituximab is a chimeric anti-CD20 monoclonal antibody, approved for diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis. The initial dosing regimen of rituximab in patients with indolent non-Hodgkin lymphoma, supported by 2 phase 1 dose-escalation trials,1,2 was 375 mg/m2 weekly for 4 consecutive weeks. From these studies, neither dose-response relationship nor dose-limiting toxicities were identified, and the reasons for selecting 375 mg/m2 infusions remain unclear.3 In studies of immunochemotherapy in DLBCL, rituximab was administered at the dose of 375 mg/m2 often in 2- or 3-week schedules. Increasing the frequency of 375 mg/m2 rituximab infusions did not result in better outcomes in all patients as compared with the classical regimen.4 Thus, dosing strategies independent of patients’ characteristics might not be sufficient, and better strategies may consist of individualizing the dosing regimen.
As for most drugs, a standard dose of rituximab leads to highly variable clinical response, with few patients (6%) reaching complete remission, as observed in pivotal studies.5,6 This variability is partly explained by rituximab pharmacokinetic (PK) variability,7-9 higher rituximab concentrations being associated with a better clinical response.10-14 Target antigen burden is one of the potential sources of rituximab PK variability. The pivotal study in patients with recurrent low-grade lymphoma reported an inverse relationship between rituximab serum levels and both tumor volume and lymphocyte count at baseline.8 In mice xenografted with human CD20-expressing tumor, rituximab clearance increased with tumor volume.12 Hence, standard doses of rituximab and a high tumor volume may lead to low rituximab serum concentrations, resulting in an insufficient therapeutic response. Rituximab population PK was previously assessed in patients with DLBCL,13,14 but the influence of tumor burden has never been investigated in these patients despite clinical evidence suggesting worse outcome in those exhibiting bulky tumor.15,16
The objectives of the present study were therefore to quantify the impact of total metabolic tumor volume (TMTV0) measured on baseline (TMTV0) using 18F-fluorodeoxyglucose-positron emission tomography-computed tomography (FDG-PET/CT) on rituximab PK, concentration-response relationship and prognosis in DLBCL patients treated with standard doses of rituximab, and to propose an individual rituximab dose adjustment according to TMTV0.
Patients and methods
Patients and treatment
Rituximab PK and clinical response data were obtained from 2 prospective, multicenter, randomized studies—LNH2007-3B and GOELAMS 02.03—conducted by the former Groupe d'Etude des Lymphomes de l'Adulte and Groupe Ouest Est d'Etude des Leucemies Aigues et Maladies du Sang (GOELAMS), which have recently merged as the Lymphoma Study Association group. These studies were approved by ethical committees and registered on ClinicalTrials.gov as NCT00498043 (LNH2007-3B) and NCT00841945 (GOELAMS 02.03). Briefly, eligible patients were aged 18 to 59 years and 18 to 75 years, respectively, with previously untreated and histologically proven CD20+ DLBCL (World Health Organization classification), receiving a first-line treatment of rituximab in combination with chemotherapy. High-risk patients with an age-adjusted international prognostic index (IPI) of 2 or 3 were eligible for the LNH2007-3B trial, whereas the GOELAMS 02.03 study recruited patients with Ann Arbor stage I or II with a tumor mass <7 cm. Patients were excluded if they had a diagnosis or history of other types of lymphoma or malignancies, HIV infection, or contraindications to rituximab or drugs contained in the chemotherapy regimens. The aim of the LNH2007-3B study was to evaluate the complete response rate according to FDG-PET/CT criteria after 4 cycles of rituximab, doxorubicin, cyclophosphamide, vincristine, bleomycine, and prednisone (R-ACVBP-14) or rituximab, doxorubicin, cyclophosphamide, vincristine, and prednisone (R-CHOP-14). The consolidation treatment was allocated based on centrally reviewed PET assessment after induction immunochemotherapy, and patients received either consolidative immunochemotherapy or high-dose therapy.17 In the GOELAMS 02.03 study, induction therapy consisted of 4 cycles of R-CHOP-14. If complete response according to FDG-PET/CT after induction therapy was achieved, patients with modified IPI = 0 were randomized to receive or not radiotherapy, whereas those with modified IPI ≥1 received 2 additional R-CHOP-14 cycles. Patients in partial response received 2 additional R-CHOP-14 cycles followed by radiotherapy.18 In both studies, rituximab was administered as 4 infusions of 375 mg/m2 repeated every 2 weeks in the induction phase. At the time of enrollment, all patients signed written informed consent and an additional informed consent specific for the PK protocol.
Rituximab concentrations
Blood samples for rituximab serum concentration measurements were collected just before and 2 hours after each rituximab infusion during the 4 cycles of induction treatment in both studies. Additional samples were drawn in the LNH2007-3B study in 30 patients of each arm on day 5 of each induction cycle, and on time of FDG-PET/CT after the fourth induction cycle (PET4). In the GOELAMS 02.03 trial, additional samples were drawn on day 21 or on the evaluation day of cycle 4 and on an additional day of cycles 1 and 4. Rituximab concentrations were measured using an enzyme-linked immunosorbent assay derived from the technique of Blasco et al.19 The limit of detection was 0.06 mg/L and the lower and upper limits of quantification were 0.20 mg/L and 7.0 mg/L, respectively.
TMTV0 measurement
FDG-PET/CT was performed before treatment. TMTV0 was computed on a semiautomatic Food and Drug Administration–approved software, Imagys (Keosys, Saint-Herblain, France). Lesions were identified by visual assessment, with PET images scaled to a fixed standardized uptake value (SUV) display and color table. TMTV0 was obtained by summing metabolic volumes of all local nodal and extranodal lesions, which were computed using the 41% maximum SUV threshold method, as recommended by the European Association of Nuclear Medicine.20 A volume of interest was set around each lesion (node or organ involvement), as previously described.21 Several volumes of interest could be drawn in bulky regions in case of heterogeneity in SUV distribution.21 Bone marrow involvement was included in volume measurement only if there was focal uptake. Spleen was considered as involved if there was focal uptake or diffuse uptake higher than 150% of the liver background. This method was demonstrated to be highly reproducible between observers and centers even when different software was used.22
Evaluation of response to treatment
In the LNH20073B trial, 2 PET examinations at mid and end of induction were required and scheduled 2 weeks after the second and fourth cycles of immunochemotherapy, respectively. In the GOELAMS 02.03 trial, PET was performed after the fourth cycle of R-CHOP-14 only. A blinded central review in real time of the PET images was organized using the POSITOSCOPE network.23 PET data were interpreted by at least 2 of 3 PET experts. PET scans were binary interpreted as positive or negative, as recommended by the International Harmonization Project,24 in which positive residual uptake should be at least 25% higher than the reference background. In both trials, the response to treatment was classified on PET4 as complete metabolic response (CMR) if PET4 was judged negative and non-CMR otherwise.
PK modeling
Rituximab PK was assessed with a population approach using Monolix 4.3.2 (Lixoft, Orsay, France). As previously reported for rituximab PK,13,25 a structural 2-compartment model best described the concentration data (see supplemental Methods, available on the Blood Web site). Rituximab exposure was measured using area under the concentration-time curve (AUC), computed using individual PK parameters. The association between TMTV0 and rituximab exposure was assessed. Several factors, including sex, age, body size, and TMTV0, were tested as covariates on PK model parameters. In the multivariate analysis, significant covariates for P < .01 were retained in the model (supplemental Methods).
Association among tumor volume, rituximab exposure, and patient outcome
Progression-free survival (PFS) was defined as the time from inclusion to progression, relapse or death from any cause. Overall survival (OS) was defined as the time from inclusion to death from any cause. PFS and OS were estimated using Kaplan-Meier analysis and compared between patient groups using the log-rank test. The influence of rituximab exposure on PET4 response, as well as on PFS and OS, was evaluated using logistic regression analysis and Cox proportional hazards regression models, respectively. TMTV0, sex, IPI score (0-2 vs 3-4), age, and associated chemotherapy (CHOP vs ACVBP) were also assessed as predictors of outcome. Results from Cox regression and logistic regression analyses were internally validated using bootstrap procedure,26,27 generating a total of 100 replicates. Optimal threshold cutoffs for prediction of PET4 response, PFS, and OS were identified with receiver operating characteristic (ROC) curve analysis and validated using bootstrap analysis (1000 replicates). P values <.05 (two-sided test) were considered to indicate statistical significance in the univariate and multivariate analyses. When variables were redundant, the most significant was kept in the multivariate analysis to avoid multicollinearity. Statistical analysis was conducted using R Software, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and survival analysis was conducted using SAS, version 9.43 (SAS Institute Inc., Cary, NC).
Individualized rituximab dose estimation
Concentration-time profiles of the first induction cycle were computed based on individual PK parameters. Doses needed to obtain the optimal AUC were calculated for TMTV0 ranging from 1 to 4340 cm3.
Results
Patient characteristics
Data on rituximab concentrations and TMTV0 were available from 108 patients (89 in the LNH2007-3B trial; 19 in the GOELAMS 02.03 trial) who were included in the PK analysis (Figure 1, Table 1). Sixty-three patients (58%) were male, with a median age of 49 years (range, 19-68 years). Patients in the LNH2007-3B trial had higher median TMTV0 (461 cm3; range, 17-4339 cm3) as compared with the GOELAMS 02.03 study (11.8 cm3; range, 0.8-75.8 cm3). Survival analysis was performed on the 108 patients assessed for PK (Figure 1). In 11 of these 108 patients, PET4 response was not evaluated; thus, 97 patients were included in the response analysis (Figure 1).
Summary of patients’ characteristics at baseline
| Patients characteristics . | LNH2007-3B (n = 89) . | GOELAMS 02.03 (n = 19) . | All . |
|---|---|---|---|
| Men/women, n | 56/33 | 7/12 | 63/45 |
| Age, y | 47 [35-55] | 54 [44-57] | 49 [35-56] |
| Weight, kg | 74 [63-83] | 71 [58-80] | 73.5 [63.0-82.2] |
| Body surface area, m2 | 1.88 [1.74-2.03] | 1.80 [1.65-1.88] | 1.85 [1.68-2.00] |
| Ann Arbor stage, n (%) | |||
| I/II | 3 (3) | 19 (100) | 22 (20) |
| III/IV | 86 (97) | 0 (0) | 86 (80) |
| IPI, n (%) | |||
| 0 | 0 (0) | 15 (79) | 15 (14) |
| 1-2 | 29 (33) | 4 (21) | 33 (31) |
| 3-4 | 60 (67) | 0 (0) | 60 (55) |
| Associated chemotherapy | |||
| CHOP, n (%) | 50 (56) | 19 (100) | 69 (64) |
| ACVBP, n (%) | 39 (44) | 0 | 39 (36) |
| TMTV0, cm3 | 461 [164-796] | 11.8 [4.0-30.8] | 313.5 [83.2-670.7] |
| Baseline lymphocytes, Giga/L | 12.9 [7.3-21.0] | 1.7 [1.4-2.0] | 9.5 [4.0-16.9] |
| CMR, n (%) | 41/88 (47) | 8/9 (89) | 49/97 (50.5) |
| AUC1, mg × h per liter | 9 497 [8 920-10 250] | 16 040 [10 640-20 940] | 9 743 [8 967-10 800] |
| Non-CMR, n (%) | 47/88 (53) | 1/9 (11) | 48/97 (49.5) |
| AUC1, mg × h per liter | 8 730 [7 973-9 592] | 9 493 | 8 733 [7 989-9 577] |
| Patients characteristics . | LNH2007-3B (n = 89) . | GOELAMS 02.03 (n = 19) . | All . |
|---|---|---|---|
| Men/women, n | 56/33 | 7/12 | 63/45 |
| Age, y | 47 [35-55] | 54 [44-57] | 49 [35-56] |
| Weight, kg | 74 [63-83] | 71 [58-80] | 73.5 [63.0-82.2] |
| Body surface area, m2 | 1.88 [1.74-2.03] | 1.80 [1.65-1.88] | 1.85 [1.68-2.00] |
| Ann Arbor stage, n (%) | |||
| I/II | 3 (3) | 19 (100) | 22 (20) |
| III/IV | 86 (97) | 0 (0) | 86 (80) |
| IPI, n (%) | |||
| 0 | 0 (0) | 15 (79) | 15 (14) |
| 1-2 | 29 (33) | 4 (21) | 33 (31) |
| 3-4 | 60 (67) | 0 (0) | 60 (55) |
| Associated chemotherapy | |||
| CHOP, n (%) | 50 (56) | 19 (100) | 69 (64) |
| ACVBP, n (%) | 39 (44) | 0 | 39 (36) |
| TMTV0, cm3 | 461 [164-796] | 11.8 [4.0-30.8] | 313.5 [83.2-670.7] |
| Baseline lymphocytes, Giga/L | 12.9 [7.3-21.0] | 1.7 [1.4-2.0] | 9.5 [4.0-16.9] |
| CMR, n (%) | 41/88 (47) | 8/9 (89) | 49/97 (50.5) |
| AUC1, mg × h per liter | 9 497 [8 920-10 250] | 16 040 [10 640-20 940] | 9 743 [8 967-10 800] |
| Non-CMR, n (%) | 47/88 (53) | 1/9 (11) | 48/97 (49.5) |
| AUC1, mg × h per liter | 8 730 [7 973-9 592] | 9 493 | 8 733 [7 989-9 577] |
Results are given as median [interquartile range].
TMTV0 correlated with rituximab exposure and elimination half-life
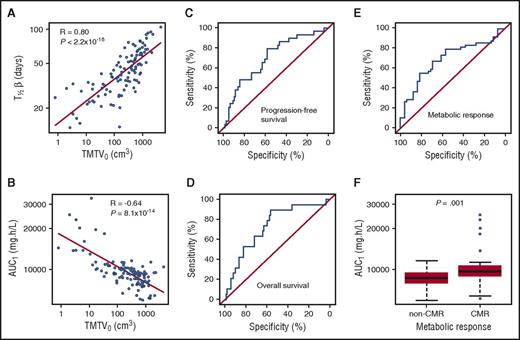
Rituximab elimination half-life (T1/2β) was 45 days in a subject with median body surface area, and increased with TMTV0 (Spearman R2 = 0.64; P < 2.2 × 10−16; Figure 2A), ranging from 12.7 days for the lowest TMTV0 of 0.8 to 83.8 days for the highest TMTV0 of 4339 cm3. An inverse correlation was found between TMTV0 and rituximab exposure before the second injection (AUC1; Spearman R2 = 0.41; P = 8.1 × 10−14; Figure 2B).
Relationships between total metabolic tumor volume, rituximab pharmacokinetics, and patient outcome. (A) T1/2β vs TMTV0. (B) Rituximab exposure in induction cycle 1 (AUC1) vs TMTV0. ROC curves of the predictive value of AUC1 for (C) PFS, (D) OS, and (E) PET4 metabolic response. Corresponding ROC AUCs were 0.73, 0.70, and 0.69, respectively. (F) AUC1 in CMR vs non-CMR (8733 vs 9743 mg × h per liter; P = .001).
Relationships between total metabolic tumor volume, rituximab pharmacokinetics, and patient outcome. (A) T1/2β vs TMTV0. (B) Rituximab exposure in induction cycle 1 (AUC1) vs TMTV0. ROC curves of the predictive value of AUC1 for (C) PFS, (D) OS, and (E) PET4 metabolic response. Corresponding ROC AUCs were 0.73, 0.70, and 0.69, respectively. (F) AUC1 in CMR vs non-CMR (8733 vs 9743 mg × h per liter; P = .001).
Rituximab exposure correlated with PET4 response and survival
Optimal predictive AUC1 cutoff using the Youden index28 was 9400 mg × h per liter for both PFS and OS with ROC AUCs of 0.70 (95% confidence interval [CI], 0.60-0.81; P = .0003) and 0.73 (95% CI, 0.61-0.85; P = .0002), respectively (Figure 2C-D). Sensitivity and specificity were 79% and 58% for PFS and 89% and 56% for OS. Using the Youden index, optimal predictive AUC1 value for PET4 response was estimated at 9600 mg × h per liter, with sensitivity and specificity of 55% and 81%, respectively (Figure 2E). An AUC1 value of 9400 mg × h per liter, identical to that for PFS and OS, resulted in sensitivity and specificity of 61% and 69%, respectively. Thus, the sum of sensitivity and specificity was only slightly different between these 2 AUC1 cutoff values. Therefore, the AUC1 of 9400 mg × h per liter was used as the optimal value for subsequent analyses for outcome and PET4 response. The median AUC1 cutoff values obtained after bootstrap analysis were nearly identical to the 9400 mg × h per liter optimal value derived from the original data set.
CMR after 4 cycles of immunochemotherapy was 50.5% (49 of 97 patients). Compared with nonresponders, CMR patients had significantly higher rituximab exposure before the second cycle (AUC1, 8733 vs 9743 mg × h per liter; Mann-Whitney P = .001; Figure 2F). In the univariate logistic regression analysis, AUC1 ≥9400 mg × h per liter, higher age, lower TMTV0, and IPI score of 0 to 2 were associated with better response with no significant influence of associated chemotherapy (Table 2). Because AUC1 and TMTV0 were correlated (Figure 2B), the most significant parameter, AUC1, was selected for the multivariate analysis. In the multivariate analysis, IPI score and chemotherapy did not influence response, whereas AUC1 (odds ratio, 5.56; 95% CI, 2.19-15.62; P = .0006) and age (odds ratio, 1.06; 95% CI, 1.02-1.10; P = .004) were the only factors associated with response (Table 2). Parameters of the final model were confirmed by the bootstrap procedure (supplemental Table 2), which indicates the stability of the model. Age, however, was unable to discriminate between CMR and non-CMR patients (ROC AUC, 0.60; 95% CI, 0.49-0.72; P = .062).
Logistic regression analysis for predictors of response according to PET4 criteria in rituximab-treated DLBCL patients (n = 97)
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Univariate analysis | |||
| AUC1 ≥9400 mg × h per liter | 3.47 | 1.52-8.21 | .004 |
| TMTV0, cm3 (×10−2) | 0.91 | 0.82-0.98 | .033 |
| IPI (3-4) | 0.36 | 0.15-0.82 | .017 |
| Chemotherapy (CHOP) | 0.95 | 0.42-2.14 | .90 |
| Male | 0.56 | 0.24-1.27 | .17 |
| Age, y | 1.04 | 1.003-1.08 | .036 |
| Final model* | |||
| AUC1 ≥9400 mg × h per liter | 5.56 | 2.19-15.62 | .0006 |
| Age, y | 1.06 | 1.02-1.10 | .004 |
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Univariate analysis | |||
| AUC1 ≥9400 mg × h per liter | 3.47 | 1.52-8.21 | .004 |
| TMTV0, cm3 (×10−2) | 0.91 | 0.82-0.98 | .033 |
| IPI (3-4) | 0.36 | 0.15-0.82 | .017 |
| Chemotherapy (CHOP) | 0.95 | 0.42-2.14 | .90 |
| Male | 0.56 | 0.24-1.27 | .17 |
| Age, y | 1.04 | 1.003-1.08 | .036 |
| Final model* | |||
| AUC1 ≥9400 mg × h per liter | 5.56 | 2.19-15.62 | .0006 |
| Age, y | 1.06 | 1.02-1.10 | .004 |
OR, odds ratio.
Final model includes significant covariates in the multivariate analysis.
With a median follow-up of 48 months (95% CI, 43-51), 4-year PFS was 76% and 4-year OS was 82%. Patients with an AUC1 ≥9400 mg × h per liter cutoff (n = 55; 51%) had a significantly better 4-year PFS (83% vs 62%; log-rank P = .011) and 4-year OS (94% vs 69%; log-rank P = .0016) than those with AUC1 under the cutoff value (Figure 3). Univariate Cox regression analysis showed longer PFS for higher AUC1, lower TMTV0, and in women, and longer OS for higher AUC1 (Table 3). No significant changes in OS or PFS were observed according to IPI or chemotherapy. Between AUC1 and TMTV0, AUC1 was selected for multivariate analysis given the significant correlation between the 2 variables (Figure 2B). In the multivariate analysis, an AUC1 ≥9400 mg × h per liter was associated with longer PFS (hazard ratio [HR], 0.38; 95% CI, 0.17-0.83; P = .011) and OS (HR, 0.17; 95% CI, 0.05-0.60; P = .001). The proportional hazards assumption was satisfied in the final model for both PFS (P = .23) and OS (P = .58). Using the bootstrap validation procedure, the multivariate Cox analysis for PFS and OS included AUC1 with nearly similar parameter estimates (supplemental Tables 3 and 4).
Kaplan-Meier estimates of PFS and OS by rituximab exposure in cycle 1 (AUC1). Patients with AUC1 ≥9400 mg × h per liter had significantly better (A) 4-year PFS (83% vs 62%; log-rank P = .011) and (B) 4-year OS (94% vs 69%; log-rank P = .0016) than those with AUC1 <9400 mg × h per liter.
Kaplan-Meier estimates of PFS and OS by rituximab exposure in cycle 1 (AUC1). Patients with AUC1 ≥9400 mg × h per liter had significantly better (A) 4-year PFS (83% vs 62%; log-rank P = .011) and (B) 4-year OS (94% vs 69%; log-rank P = .0016) than those with AUC1 <9400 mg × h per liter.
Cox regression analysis for PFS and OS in rituximab-treated DLBCL patients (n = 108)
| . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Univariate analysis | ||||||
| AUC1 ≥9400 mg × h per liter | 0.38 | 0.17-0.83 | .011 | 0.17 | 0.05-0.60 | .001 |
| TMTV0, cm3 (×10−2) | 1.07 | 1.02-1.13 | .005 | 1.03 | 0.98-1.09 | .24 |
| IPI (3-4) | 2.01 | 0.91-4.42 | .082 | 2.14 | 0.76-6.00 | .15 |
| Chemotherapy (CHOP) | 0.81 | 0.39-1.70 | .577 | 1.09 | 0.41-2.90 | .87 |
| Male | 2.42 | 1.03-5.66 | .042 | 2.30 | 0.81-6.48 | .11 |
| Age, y | 1.02 | 0.99-1.05 | .262 | 1.03 | 0.99-1.08 | .17 |
| Final model with exposure* | ||||||
| AUC1 ≥9400 mg × h per liter | 0.38 | 0.17-0.83 | .011 | 0.17 | 0.05-0.60 | .001 |
| . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Univariate analysis | ||||||
| AUC1 ≥9400 mg × h per liter | 0.38 | 0.17-0.83 | .011 | 0.17 | 0.05-0.60 | .001 |
| TMTV0, cm3 (×10−2) | 1.07 | 1.02-1.13 | .005 | 1.03 | 0.98-1.09 | .24 |
| IPI (3-4) | 2.01 | 0.91-4.42 | .082 | 2.14 | 0.76-6.00 | .15 |
| Chemotherapy (CHOP) | 0.81 | 0.39-1.70 | .577 | 1.09 | 0.41-2.90 | .87 |
| Male | 2.42 | 1.03-5.66 | .042 | 2.30 | 0.81-6.48 | .11 |
| Age, y | 1.02 | 0.99-1.05 | .262 | 1.03 | 0.99-1.08 | .17 |
| Final model with exposure* | ||||||
| AUC1 ≥9400 mg × h per liter | 0.38 | 0.17-0.83 | .011 | 0.17 | 0.05-0.60 | .001 |
Final model includes significant covariates in the multivariate analysis.
Nomogram for dose individualization
Based on the final model simulations, the optimal dose ( ) to administer to a patient according to individual TMTV0 value and resulting in the optimal AUC1 of 9400 mg × h per liter can be written as:
) to administer to a patient according to individual TMTV0 value and resulting in the optimal AUC1 of 9400 mg × h per liter can be written as:  (supplemental Results). For instance, the standard dose of 375 mg/m2 would be suitable for patients with TMTV0 below 281 cm3. For the median TMTV0 value in the population of 313.5 cm3 (median value in the present study), a dose of 378 mg/m2 would be required to achieve optimal exposure. A patient with a TMTV0 of 4339 cm3 (highest value in this study) would require a dose of 468 mg/m2 (supplemental Table 5). Finally, if we applied individualized dosing, the total dose of rituximab administered in the first immunochemotherapy cycle in patients potentially underexposed (TMTV0 > 281 cm3, n = 56 of 97) would have been 23 140 mg/m2 instead of 21 000 mg/m2 (supplemental Table 5), followed by the 375 mg/m2 dose per cycle in subsequent cycles.
(supplemental Results). For instance, the standard dose of 375 mg/m2 would be suitable for patients with TMTV0 below 281 cm3. For the median TMTV0 value in the population of 313.5 cm3 (median value in the present study), a dose of 378 mg/m2 would be required to achieve optimal exposure. A patient with a TMTV0 of 4339 cm3 (highest value in this study) would require a dose of 468 mg/m2 (supplemental Table 5). Finally, if we applied individualized dosing, the total dose of rituximab administered in the first immunochemotherapy cycle in patients potentially underexposed (TMTV0 > 281 cm3, n = 56 of 97) would have been 23 140 mg/m2 instead of 21 000 mg/m2 (supplemental Table 5), followed by the 375 mg/m2 dose per cycle in subsequent cycles.
Discussion
To our knowledge, this is the first study describing the influence of total metabolic tumor volume (TMTV) on rituximab PK and concentration-response relationship in patients with DLBCL. Patients with higher tumor burden had lower exposure to rituximab, which was associated with worse clinical response and shorter survival. Based on initial TMTV, individualized rituximab dose leading to optimal exposure and thereby optimal outcome was then predicted.
Rituximab PK was satisfactorily described using a 2-compartment model as in previous studies.13,14,25,29-31 We observed a decrease in exposure to rituximab with increasing TMTV0. Similarly, rituximab serum levels were previously shown to correlate inversely with tumor bulk at baseline in low-grade lymphoma.8 This may be explained by increased rituximab clearance related to its elimination by the target antigen. Daydé et al12 also demonstrated an increase in rituximab elimination with tumor increase in a murine model of disseminated lymphoma. This phenomenon was previously described for several monoclonal antibodies and is known as target-mediated drug disposition.31-34 In contrast to these results, we showed in the present study that higher TMTV0 was associated with increased rituximab volumes of distribution without modifying clearance (supplemental Results). This may be due to increased retention of rituximab by the tumor, followed, after treatment discontinuation, by a slower return of rituximab to the circulation. Indeed, this slower release with increasing TMTV0 is confirmed by higher rituximab elimination half-life, reaching 12 weeks for TMTV0 of 4339 cm3. Median half-life was 6.4 weeks, a value longer than that reported in follicular lymphoma (∼3 weeks29 ). Similarly, Muller et al13 reported a prolonged rituximab terminal half-life of 5 weeks in DLBCL. However, the aforementioned study did not investigate the potential influence of tumor volume on rituximab PK as a factor that might be responsible for this prolonged half-life.
In our study, patient outcome assessed by PET4 response, PFS, and OS was associated with rituximab exposure, and improved outcome was observed for higher AUC1. These findings are in agreement with other studies in which higher rituximab concentrations were found in responders in both indolent lymphoma8,11 and aggressive lymphoma.7,10 Igarashi et al9 also observed a longer time to progression in patients with higher serum rituximab levels.
Tumor burden was previously shown to be associated with clinical outcome, where TMTV0 evaluated by 18F-FDG-PET/CT was reported as an independent predictor of OS in DLBCL patients treated by immunochemotherapy, with shorter OS in the high TMTV group.16 Similarly, Pfreundschuh et al15 observed worse OS for DLBCL patients treated by immunochemotherapy exhibiting bulky tumor. In our study, we found that tumor burden explained a large part of the variability in rituximab exposure, TMTV0 accounting for 41% of AUC1 variability. Because higher exposure to rituximab is associated with improved outcome, TMTV0 may be considered as a predictor of adequate exposure to rituximab.
Previous studies attempted to optimize rituximab treatment by testing different dosing regimens.7,13,35 Higher frequency of 375 mg/m2 rituximab infusions did not result in better outcomes in all patients as compared with the classical regimen.4,36 The SEXIE-R-CHOP-14 study demonstrated that an increased rituximab dose of 500 mg/m2 in elderly male patients resulted in improved outcome.37 However, the same dose might not be appropriate for all DLBCL patients. Rituximab efficacy is influenced by factors other than patient sex. Our findings demonstrating a clear influence of tumor volume on rituximab PK and hence on patient outcome, in addition to previous studies in line with ours, suggest the benefit of adapting rituximab dose to TMTV0.
In the present study, we propose individualizing rituximab dose based on TMTV0 to achieve optimal exposure and thereby improved outcome. Associations between rituximab AUC and response were significant for induction cycles 1, 2, and 3 (data not shown) with no significant differences between the corresponding ROC AUCs. Hence, subsequent analyses were based on the earlier AUC of first cycle. An optimal predictive AUC1 value of 9400 mg × h per liter was used for clinical response, PFS, and OS. Rituximab dose that would achieve optimal exposure in a patient with a given TMTV0 can be calculated as given previously in “Nomogram for dose individualization.” For patients with the highest TMTV0 of 4339 cm3, an increase of 25% (468 mg/m2) over the standard dose would then be necessary to reach the optimal exposure. The standard 375 mg/m2 dose would be appropriate for patients with a TMTV0 of 281 cm3. Median TMTV0 in the present study was 313.5 cm3, and 52% of patients (TMTV0 >281 cm3) were potentially underexposed to rituximab. Previous studies in DLBCL16,38-40 reported median TMTV0 around 300 cm3, indicating that more than 50% of patients had a TMTV0 higher than the 281 cm3 value. Although these data were not all obtained in series of patients with the same characteristics or measured using the same techniques, this suggests that a considerable proportion of DLBCL patients would benefit from this dose individualization.
Doses >375 mg/m2 of rituximab given as monotherapy were previously evaluated in aggressive B-cell lymphoma, and the 500 mg/m2 dose seemed to be well tolerated.41 A 500 mg/m2 dose of rituximab in association with chemotherapy was not associated with increased toxicities in male patients in the SEXIE-R-CHOP-14 study. Safety of rituximab doses >375 mg/m2, administered with chemotherapy, particularly in patients with high tumor volume, remains to be investigated, however. Furthermore, a dose of 500 mg/m2 for all patients would lead to an overexposure of 100% of our patients, which would also induce an extra cost of 33%; this extra cost could be evaluated according to our nomogram to be a mean of 0.11% in our population (from −33% to +25% according to individual TMTV0). Thus, dose-adapted approaches would represent a better use of resources.
The proposed dose individualization of rituximab in the present study was done retrospectively. The benefit of this dosing strategy therefore needs to be confirmed in a prospective clinical trial testing the adaptation of rituximab dose according to patients’ baseline metabolic tumor volume vs the standard dosing regimen.
Although the population included in our study is younger than the general DLBCL population, it is of high similarity with DLBCL population in terms of the metabolic tumor volume. For instance, previously reported median TMTV0 values in DLBCL were around 300 cm3,16,39 which are very similar to the median value in our population.
Overall, this study is the first to quantify the influence of metabolic tumor volume on rituximab PK in patients with DLBCL. We showed that rituximab exposure decreased with increasing baseline tumor volume. Rituximab exposure was found to be predictive of response after induction treatment, OS, and PFS. Our work allowed us to design a nomogram giving the optimal dose to administer according to individual TMTV0. These results should lead to a design of future clinical trials testing the benefit of individual adjustment of rituximab dose to TMTV0.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anne-Claire Duveau, Caroline Guerineau-Brochon, and Céline Desvignes for technical assistance, and Nicolas Azzopardi for pharmacokinetic advice.
Measurements of rituximab serum concentrations were carried out within the Centre Pilote de suivi biologique des Anticorps thérapeutiques (CePiBAc) platform. CePiBAc was cofinanced by the European Union. Europe is committed to the region Centre with the European Regional Development Fund. This work was supported by the French Higher Education and Research Ministry under the program “Investissements d’avenir” Grant Agreement: LabEx MAbImprove ANR-10-LABX-53-01. This study was funded by the Groupe d'Etude des Lymphomes de l’Adulte, Groupe Ouest Est d'Etude des Leucemies Aigues et Maladies du Sang, and F. Hoffman-La Roche Ltd (Basel, Switzerland).
Authorship
Contribution: M.T. collected, analyzed, and interpreted data, performed pharmacokinetic and statistical analyses, and wrote the manuscript; O.C. designed research, collected, analyzed, and interpreted data, and wrote the manuscript; M. Meignan, T.L., F.M., G.S., E.G., C.H., M. Mercier, and P.F. contributed study materials/patients, collected, analyzed, and interpreted data, and wrote the manuscript. T.L., F.M., G.S., E.G., C.H., M. Mercier, and P.F. contributed study materials/patients, collected, analyzed, and interpreted data, and reviewed the manuscript; S.B. collected, analyzed, and interpreted data, performed statistical analysis, and reviewed the manuscript; G.P. designed research, analyzed and interpreted data, and reviewed the manuscript; D.T. designed research, analyzed and interpreted data, and wrote the manuscript; and G.C. designed research, contributed study materials/patients, collected, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: O.C. received honoraria for participation in advisory boards organized by Roche and received research funding from Roche. F.M. received honoraria for participation in advisory boards or scientific meetings organized by Roche. G.S. received honoraria for participation in advisory boards or scientific meetings organized by Roche and received a research grant from Roche. E.G. received research funding from Roche, is a coordinating investigator of a clinical trial supported by Roche, and received honoraria for scientific meetings organized by Roche. C.H. received honoraria for participation in advisory boards organized by Roche and received research funding from Roche. G.P. received research funding from Novartis, Roche Pharma, Genzyme, MSD, Chugai, and Pfizer outside of the submitted work. D.T. has given lectures for Amgen and Sanofi. G.C. received consultancy fees and honoraria from Roche. The remaining authors declare no competing financial interests.
Correspondence: Guillaume Cartron, Département d’Hématologie Clinique, Centre Hospitalier Régional Universitaire, 80 Avenue Augustin Fliche, 34095 Montpellier Cedex 05, France; e-mail: g-cartron@chu-montpellier.fr.