To the editor:
Acute promyelocytic leukemia (APL) is characterized by the promyelocytic leukemia-retinoic acid receptor α (PML-RARA) fusion. In rare instances, RARA is fused to other partners, which dictate sensitivity to targeted therapies. Chen et al previously reported in Blood a novel TBLR1-RARA fusion, which is all-trans-retinoic acid (ATRA)-insensitive in vivo, in a t(3;17)(q26;q21)-harboring APL.1,2 Here, we report another new RARA fusion resulting from the same translocation in a variant APL patient.
The patient was a 36-year-old man who presented with fatigue, dyspnea, and easy bruising for 2 weeks. Complete blood count revealed a hemoglobin level of 5.4 g/dL, platelet count of 41 × 109/L, and white blood cell count of 3.6 × 109/L with 60% hypergranular blasts. Clotting profile showed a decreased fibrinogen level and prolonged prothrombin time but normal activated partial thromboplastin time. Bone marrow (BM) examination showed 68% of blasts with morphology similar to those in peripheral smear (Figure 1A). The blasts were positive for myeloperoxidase, CD13, CD15, CD33, and CD117 but negative for CD34 and HLA-DR by flow cytometry. A diagnosis of APL was suggested and ATRA (45 mg/m2 per day) was initiated while awaiting molecular findings. On day 4 of ATRA therapy, the patient developed differentiation syndrome (DS) with fluid retention and pleural effusions. Steroids and diuretics were started, and the 7 + 3 induction chemotherapy was commenced with cytarabine (200 mg/m2) and daunorubicin (60 mg/m2). A morphological complete remission was confirmed at day 30.
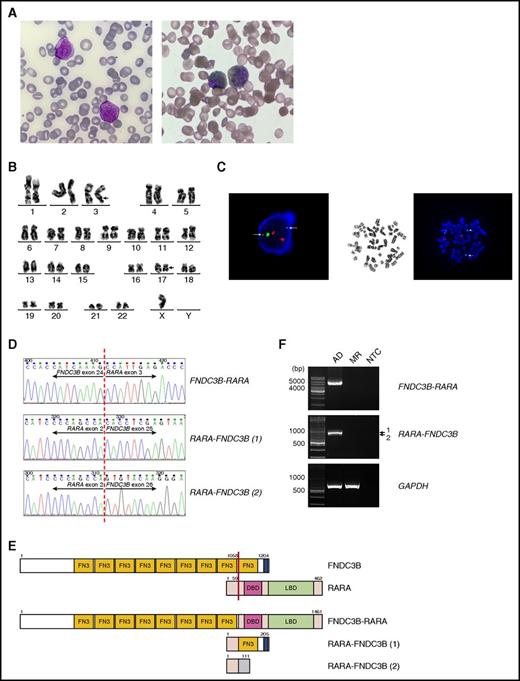
Identification of FNDC3B as a new fusion partner of RARA in a variant APL. (A) Left, May-Grünwald-Giemsa staining showing 2 abnormal promyelocytes in the diagnostic BM aspirate. The abnormal cells in the BM were medium-sized and showed round, indented, or occasionally bilobed nuclei, dispersed chromatin, prominent nucleoli, and abundant heavily granulated cytoplasm. Auer rod was rarely found. Right, Sudan Black B (SBB) staining on BM aspirate showing 2 SBB-positive abnormal promyelocytes. Original magnification ×1000. (B) A karyotype performed on the diagnostic BM revealed 45,X,-Y,t(3;17)(q26;q21). The breakpoint regions of derivative chromosomes 3 and 17 are marked with arrows. (C) Left, Interphase FISH using the PML/RARA dual-color dual-fusion translocation probes revealed splitting of the RARA gene. Two diminished split RARA signals (green) are marked with arrows. Right, Metaphase FISH analysis confirmed 3′-RARA location at 3q26 (arrowhead). 5′-RARA remained at the long arm of derivative chromosome 17 (arrow). (D) Partial nucleotide sequences surrounding the junctions of the FNDC3B-RARA and RARA-FNDC3B fusions. RARA-FNDC3B (1) and RARA-FNDC3B (2) represent the major and minor reciprocal fusion transcripts, respectively. (E) Schematic diagram of FNDC3B, RARA, FNDC3B-RARA, and RARA-FNDC3B fusion proteins. The breakpoint is indicated by a red line. The blue area in FNDC3B is a putative transmembrane domain. The shaded area in RARA-FNDC3B (2) indicates novel sequences resulting from an out-of-frame fusion. The numbers indicate amino acid positions. (F) RT-PCR analysis of the full-length FNDC3B-RARA and RARA-FNDC3B fusion transcripts in BM collected at diagnosis (AD) and molecular remission (MR). Amplification of GAPDH served as an internal control. DBD, DNA-binding domain; LBD, ligand-binding domain; NTC, no template control.
Identification of FNDC3B as a new fusion partner of RARA in a variant APL. (A) Left, May-Grünwald-Giemsa staining showing 2 abnormal promyelocytes in the diagnostic BM aspirate. The abnormal cells in the BM were medium-sized and showed round, indented, or occasionally bilobed nuclei, dispersed chromatin, prominent nucleoli, and abundant heavily granulated cytoplasm. Auer rod was rarely found. Right, Sudan Black B (SBB) staining on BM aspirate showing 2 SBB-positive abnormal promyelocytes. Original magnification ×1000. (B) A karyotype performed on the diagnostic BM revealed 45,X,-Y,t(3;17)(q26;q21). The breakpoint regions of derivative chromosomes 3 and 17 are marked with arrows. (C) Left, Interphase FISH using the PML/RARA dual-color dual-fusion translocation probes revealed splitting of the RARA gene. Two diminished split RARA signals (green) are marked with arrows. Right, Metaphase FISH analysis confirmed 3′-RARA location at 3q26 (arrowhead). 5′-RARA remained at the long arm of derivative chromosome 17 (arrow). (D) Partial nucleotide sequences surrounding the junctions of the FNDC3B-RARA and RARA-FNDC3B fusions. RARA-FNDC3B (1) and RARA-FNDC3B (2) represent the major and minor reciprocal fusion transcripts, respectively. (E) Schematic diagram of FNDC3B, RARA, FNDC3B-RARA, and RARA-FNDC3B fusion proteins. The breakpoint is indicated by a red line. The blue area in FNDC3B is a putative transmembrane domain. The shaded area in RARA-FNDC3B (2) indicates novel sequences resulting from an out-of-frame fusion. The numbers indicate amino acid positions. (F) RT-PCR analysis of the full-length FNDC3B-RARA and RARA-FNDC3B fusion transcripts in BM collected at diagnosis (AD) and molecular remission (MR). Amplification of GAPDH served as an internal control. DBD, DNA-binding domain; LBD, ligand-binding domain; NTC, no template control.
Reverse transcription–polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) failed to detect PML-RARA in the diagnostic BM. However, FISH indicated 72% of cells with split RARA signals, suggesting a variant RARA translocation. Karyotype and metaphase FISH studies revealed 45,X,-Y,t(3;17)(q26;q21)[8]/46,XY[5] (Figure 1B-C) but the expected TBLR1-RARA fusion previously identified in t(3;17) was absent. No mutations in FLT3, NPM1, CEBPA, DNMT3A, RUNX1, K/NRAS, WT1, or IDH1/2 were detected. Using 5′-rapid amplification of complementary DNA ends, we found that RARA was fused to another 3q26 gene called fibronectin type III (FN3) domain containing 3B (FNDC3B) in our patient. Subsequent RT-PCR confirmed the fusion between exon 24 of FNDC3B and exon 3 of RARA (Figure 1D), which is involved in all other RARA fusions. FNDC3B was originally identified as an adipocyte differentiation factor.3 It contains 9 FN3 domains, which are implicated in protein interactions. The full-length FNDC3B-RARA transcript is predicted to encode a 1461-amino acid protein, containing 8 FN3 domains of FNDC3B as well as the DNA-binding and ligand-binding domain of RARA (Figure 1E). Two reciprocal RARA-FNDC3B transcripts were also detected. The major transcript involves an in-frame fusion between RARA exon 2 and FNDC3B exon 25, whereas the minor transcript involves an out-of-frame fusion between the same RARA exon and FNDC3B exon 26 (Figure 1D). These transcripts are expected to generate 205- and 111-amino acid proteins, respectively (Figure 1E). Both FNDC3B-RARA and RARA-FNDC3B fusions were undetected after the patient had completed the consolidation treatment (5 + 2 chemotherapy with cytarabine 200 mg/m2 and daunorubicin 45 mg/m2 followed by high-dose cytarabine-based second consolidation) (Figure 1F). Eight months after starting maintenance therapy with ATRA/methotrexate/6-mercaptopurine, the disease relapsed and cytogenetic studies showed clonal evolution with 45,X,-Y,der(1) t(1;8)(q42;q21),t(3;17)(q26;q21),t(10;13)(p11.2;q12)[11]/46,XY[9]. Exome sequencing identified a germ line variant in the mismatch repair gene MSH3 that might underlie the genomic instability (supplemental Figure 1, available on the Blood Web site).
Immunofluorescence studies showed that FNDC3B-RARA was predominantly nuclear (Figure 2A; supplemental Figure 2). In contrast, FNDC3B resided in the cytoplasm. Further analysis of endogenous FNDC3B revealed its colocalization with calnexin (supplemental Figure 3), supporting its presence in the endoplasmic reticulum.4 Self-association and heterodimerization with retinoid X receptor α (RXRA) are common features of RARA fusion proteins.5 We found that FNDC3B-RARA could also dimerize with itself and RXRA (Figure 2B-C). Additionally, FNDC3B-RARA could heterodimerize with FNDC3B (supplemental Figure 4), suggesting that the fusion protein may deregulate FNDC3B-associated pathways.
Molecular characterization of FNDC3B-RARA and implications of FNDC3B in granulocytic differentiation. (A) Immunofluorescence analysis of FNDC3B-RARA and FNDC3B localization. HeLa cells were transfected with the indicated C-terminally hemagglutinin (HA)-tagged expression plasmids, and the tagged proteins were detected using an anti-HA antibody followed by an Alexa Fluor 488–conjugated secondary antibody. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. PML-RARA exhibited a microspeckled nuclear pattern as expected. Original magnification ×1000. (B) Analysis of FNDC3B-RARA homodimerization. HeLa cells were transfected with the indicated combinations of C-terminally Myc-tagged and HA-tagged FNDC3B-RARA expression vectors. pCMV-Myc and pCMV-HA are empty vectors. Myc-tagged proteins were immunoprecipitated and samples were analyzed by immunoblotting using the indicated antibodies. (C) Analysis of FNDC3B-RARA heterodimerization with RXRA. Myc-tagged FNDC3B-RARA expression vector was cotransfected with pCI-RXRA or pCI-RARA into HeLa cells. Myc-tagged proteins were immunoprecipitated and samples were analyzed by immunoblotting. FNDC3B-RARA interacts with RXRA but not RARA. In panels B and C, FR indicates FNDC3B-RARA. Twenty micrograms of cell lysates were analyzed in all input samples. (D) Unliganded FNDC3B-RARA is a potent transcriptional repressor. The Cignal RARE reporter was cotransfected with pCI-RARA (RARA), pCI-FNDC3B-RARA (FR), pCI-PML-RARA (PR), or the empty pCI vector (EV) into HeLa and U937 cells. Luciferase activities were measured 30 hours after transfection. Results are presented as relative luciferase activity by comparing to the empty vector control. **P < .01 and ***P < .0001 vs RARA, respectively. (E) The transcriptional activity of FNDC3B-RARA is strongly stimulated by ATRA. After 24 hours of cotransfection with the RARE reporter and various pCI expression vectors as described in panel D, cells were treated with the indicated concentrations of ATRA or dimethyl sulfoxide (DMSO) (vehicle control) for 6 hours before luciferase measurement. Results are presented as fold activation by comparing ATRA treatment to DMSO. *P < .05 and **P < .01, respectively. In panels D and E, transfection efficiency was normalized based on the constitutively expressing Renilla luciferase construct in the RARE reporter and results are expressed as mean ± standard error (SE) from at least 3 independent experiments each performed in triplicate. (F) FNDC3B knockdown impaired ATRA-induced differentiation of NB4 cells. Top, Confirmation of FNDC3B knockdown after 48 hours of siRNA transfection by western blotting. β-actin served as the loading control. Twenty four hours after siRNA transfection, NB4 cells were treated with 100 nM ATRA or DMSO (vehicle control) for 3 days. CD11b expression was measured by flow cytometry (middle) and CCAAT/enhancer-binding protein ε (CEBPE) expression by quantitative RT-PCR and normalized to GAPDH (bottom). Results are expressed as mean ± SE from 3 independent experiments. For CEBPE, expression levels were relative to DMSO treatment. (G) FNDC3B knockdown enhanced NB4 cell proliferation. Twenty four hours after siRNA transfection, NB4 cells were subjected to WST-1 (top) and colony-forming assays (bottom) for cell proliferation analysis. Results are expressed as mean ± SE from 3 independent experiments. In panels F-G, *P < .05 and **P < .01 vs control siRNA, respectively. IP, immunoprecipitation; RFU, relative fluorescence unit.
Molecular characterization of FNDC3B-RARA and implications of FNDC3B in granulocytic differentiation. (A) Immunofluorescence analysis of FNDC3B-RARA and FNDC3B localization. HeLa cells were transfected with the indicated C-terminally hemagglutinin (HA)-tagged expression plasmids, and the tagged proteins were detected using an anti-HA antibody followed by an Alexa Fluor 488–conjugated secondary antibody. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. PML-RARA exhibited a microspeckled nuclear pattern as expected. Original magnification ×1000. (B) Analysis of FNDC3B-RARA homodimerization. HeLa cells were transfected with the indicated combinations of C-terminally Myc-tagged and HA-tagged FNDC3B-RARA expression vectors. pCMV-Myc and pCMV-HA are empty vectors. Myc-tagged proteins were immunoprecipitated and samples were analyzed by immunoblotting using the indicated antibodies. (C) Analysis of FNDC3B-RARA heterodimerization with RXRA. Myc-tagged FNDC3B-RARA expression vector was cotransfected with pCI-RXRA or pCI-RARA into HeLa cells. Myc-tagged proteins were immunoprecipitated and samples were analyzed by immunoblotting. FNDC3B-RARA interacts with RXRA but not RARA. In panels B and C, FR indicates FNDC3B-RARA. Twenty micrograms of cell lysates were analyzed in all input samples. (D) Unliganded FNDC3B-RARA is a potent transcriptional repressor. The Cignal RARE reporter was cotransfected with pCI-RARA (RARA), pCI-FNDC3B-RARA (FR), pCI-PML-RARA (PR), or the empty pCI vector (EV) into HeLa and U937 cells. Luciferase activities were measured 30 hours after transfection. Results are presented as relative luciferase activity by comparing to the empty vector control. **P < .01 and ***P < .0001 vs RARA, respectively. (E) The transcriptional activity of FNDC3B-RARA is strongly stimulated by ATRA. After 24 hours of cotransfection with the RARE reporter and various pCI expression vectors as described in panel D, cells were treated with the indicated concentrations of ATRA or dimethyl sulfoxide (DMSO) (vehicle control) for 6 hours before luciferase measurement. Results are presented as fold activation by comparing ATRA treatment to DMSO. *P < .05 and **P < .01, respectively. In panels D and E, transfection efficiency was normalized based on the constitutively expressing Renilla luciferase construct in the RARE reporter and results are expressed as mean ± standard error (SE) from at least 3 independent experiments each performed in triplicate. (F) FNDC3B knockdown impaired ATRA-induced differentiation of NB4 cells. Top, Confirmation of FNDC3B knockdown after 48 hours of siRNA transfection by western blotting. β-actin served as the loading control. Twenty four hours after siRNA transfection, NB4 cells were treated with 100 nM ATRA or DMSO (vehicle control) for 3 days. CD11b expression was measured by flow cytometry (middle) and CCAAT/enhancer-binding protein ε (CEBPE) expression by quantitative RT-PCR and normalized to GAPDH (bottom). Results are expressed as mean ± SE from 3 independent experiments. For CEBPE, expression levels were relative to DMSO treatment. (G) FNDC3B knockdown enhanced NB4 cell proliferation. Twenty four hours after siRNA transfection, NB4 cells were subjected to WST-1 (top) and colony-forming assays (bottom) for cell proliferation analysis. Results are expressed as mean ± SE from 3 independent experiments. In panels F-G, *P < .05 and **P < .01 vs control siRNA, respectively. IP, immunoprecipitation; RFU, relative fluorescence unit.
Altered RARA transcriptional control is central to APL pathogenesis and different RARA fusion proteins have varying ATRA-induced transcriptional responses.1,5,6 Without ATRA, FNDC3B-RARA exerted more robust repression than RARA on a retinoic acid-response element–containing luciferase reporter (Figure 2D). Intriguingly, the activity of FNDC3B-RARA was strongly stimulated by ATRA and the fusion protein appeared as a more potent activator than RARA at pharmacological ATRA doses (10−6 M) (Figure 2E; supplemental Figure 5). Indeed, we found limited effects of FNDC3B-RARA on RARA-mediated transcriptional activation (supplemental Figure 6). It has been shown that RARA has dual roles in granulopoiesis with the unliganded receptor inhibiting and the liganded receptor promoting differentiation.7 Our data suggest that FNDC3B-RARA deregulates RARA transcriptional control primarily by enhancing the normal repressor function of unliganded RARA, thereby blocking APL cell differentiation. Like other RARA fusion proteins,1,5,8 we found that FNDC3B-RARA expression was downregulated by ATRA treatment (supplemental Figure 7). No discernible change in its subcellular distribution was noted. Our findings of ATRA-induced transcriptional activation and degradation of FNDC3B-RARA indicate that the fusion is ATRA-sensitive. However, despite a favorable initial ATRA response in our patient, long-term responses associated with this fusion remain to be clarified.
DS is a life-threatening complication in APL patients receiving ATRA. It has been suggested that ligand-activated PML-RARA directly induces chemokine expression in differentiating APL cells, thereby triggering DS development.9,10 Because FNDC3B-RARA is a potent transcriptional activator at pharmacological ATRA doses, it may be possible that the hyperactivated RARA signaling during ATRA therapy causes chemokine overproduction and early DS in our patient, who lacks risk factors for DS including high white blood cell counts, abnormal serum creatinine levels, and FLT3–internal tandem duplication.11
Using BloodSpot,12 we observed that FNDC3B messenger RNA expression increases during normal granulocytic differentiation, with the highest level in circulating polymorphonuclear cells. Also, FNDC3B was found to be specifically upregulated in the French-American-British M3 subtype of acute myeloid leukemia (supplemental Figure 8). Concordantly, we found that ATRA-induced differentiation of NB4 and HL-60 cells was associated with progressive FNDC3B upregulation (supplemental Figure 9), further supporting a role of the partner gene in granulocyte development. Consistently, small-interfering RNA (siRNA) knockdown of FNDC3B impaired ATRA-induced NB4 differentiation as indicated by reduced expression of CD11b and CCAAT/enhancer-binding protein ε (Figure 2F), a marker of terminal granulopoiesis.13 The impaired differentiation was also accompanied by increased G1-to-S cell cycle transition (supplemental Figure 10). Treatment of NB4 cells with FNDC3B siRNAs enhanced proliferation, which was also observed in OCI-AML3 cells (Figure 2G; supplemental Figure 11).
To our knowledge, FNDC3B-RARA is the 13th RARA fusion gene identified. This fusion is rather unusual in that the partner gene is also implicated in granulocytic differentiation. It is worth noting that rare APL cases without RARA rearrangements have been reported, implying that alternative mechanisms can mediate the promyelocytic differentiation block.14 Interestingly, FNDC3B was recently found to be disrupted in an APL-like acute myeloid leukemia patient lacking RARA rearrangements.15 Together, these findings highlight the possible involvement of FNDC3B deregulation in the pathogenesis of APL-like disease that is unrelated to RARA alterations. On the other hand, the role of RARA-FNDC3B remains unclear. The major isoform contains the ligand-independent transactivation domain (A domain) of RARA fused to the last FN3 domain of FNDC3B (Figure 1E). Because the FN3 domain lacks DNA-binding activity, the protein seems unlikely to be an aberrant transcription factor.
In summary, we identified FNDC3B-RARA as another RARA fusion with seemingly opposite ATRA responses within the t(3;17)-variant APL subtype. Our findings also revealed some unique features of FNDC3B-RARA and deciphering their roles shall provide significant insights into APL pathogenesis.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Mark D. Minden (Princess Margaret Cancer Centre, University Health Network, Toronto, Canada) for providing the OCI-AML3 cell line. The authors thank the Core Utilities of Cancer Genomics and Pathobiology (CUHK) for providing the facilities and assistance in support of this research.
Contribution: C.K.C. and A.Z.W. designed and performed research and wrote the manuscript; T.H.Y.W., T.S.K.W., J.S.C., and N.P.H.C. performed research and collected and analyzed the data; R.R. collected and analyzed clinical data; and M.H.L.N. designed research and advised on revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret H. L. Ng, Blood Cancer Cytogenetics and Genomics Laboratory, Department of Anatomical and Cellular Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, NT, Hong Kong SAR, China; e-mail: margaretng@cuhk.edu.hk.
References
Author notes
C.K.C. and A.Z.W. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal