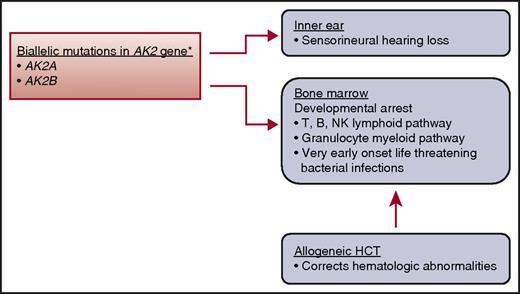
In this issue of Blood, Hoeing et al further our understanding on the clinical presentation and treatment of reticular dysgenesis (RD), a rare entity of the severe combined immunodeficiency diseases (SCIDs).1 The SCIDs are phenotypically and genotypically heterogeneous, and RD is considered 1 of the most severe forms.2,3 RD is inherited as an autosomal recessive disorder and accounts for <2% of SCID and classed as T-, B-, and natural killer–deficient SCID.2 Clinically, it is characterized by an absence of granulocytes and lymphocytes in peripheral blood, hypoplasia of the thymus and secondary lymphoid organs, lack of innate and adaptive humoral and cellular functions, and sensorineural hearing deficit.3 Unlike classical SCID, RD presents very early, usually within a few days after birth, and with life-threatening bacterial infections rather than opportunistic infections. A complete blood count with differential should alert the astute clinician to consider SCID in the differential diagnosis and undertake urgent consultation with an immunologist. Children with RD have mutations in both copies of the adenylate kinase 2 gene3 (see figure). The resulting defect in mitochondrial adenylate kinase 2 results in defective maturation of lymphoid and myeloid cells. Consequently, hematopoietic cell transplantation (HCT) is the only treatment curative for this otherwise fatal disease.
Genetic mutation, clinical characteristics, and treatment. *AK2 gene is expressed in mitochondrial space in a variety of tissues. AK2, adenylate kinase 2; NK, natural killer.
Genetic mutation, clinical characteristics, and treatment. *AK2 gene is expressed in mitochondrial space in a variety of tissues. AK2, adenylate kinase 2; NK, natural killer.
In describing the natural history of the disease, Hoenig et al note the high proportion of premature births, infants small for gestational age, life-threatening infections much earlier than seen with classical SCID, lymphopenia, and agranulocytosis. Other hematological features observed in their cohort included thrombocytopenia and hemoglobin levels below the normal range. The most common finding on examination of bone marrow morphology was arrest of myeloid differentiation at the promyelocytic stage. The majority of infants presented within the first week after birth. The authors recommend a diagnostic workup for infants with unexplained leukopenia because early referral to a specialist and HCT is potentially lifesaving. Although long-term survival after HCT was 68%, graft failure or persistence/recurrence of agranulocytosis was the predominant cause of treatment failure.1 Myeloablative transplant conditioning regimens and transplantation of T-cell–replete grafts were associated with best outcomes.1
This is an important observation because transplant strategies for classical SCID vary. For classical SCID, grafts from HLA-matched siblings or from unrelated donors are unmodified and recipients receive immunosuppression posttransplant for graft-versus-host disease prophylaxis.4 Grafts from HLA-mismatched relatives are T-cell–depleted and administered without further immunosuppression for graft-versus-host disease.4,5 Although the majority of HLA-matched and mismatched related donor transplants occur without a transplant-conditioning regimen, recipients of unrelated donor transplants receive transplant-conditioning regimens that are more likely to be reduced in their intensity.4 For several other primary immunodeficiency diseases, reduced intensity conditioning regimens with alkylating agents result in sustained engraftment and long-term survival.6 Given the rarity of RD, we have to conclude that myeloablative regimens and transplantation of T-cell–replete grafts are preferred to reduced intensity conditioning regimens. Although RD is a SCID, it is associated with other hematopoietic abnormalities, primarily agranulocytosis; this is likely why myeloablative transplant conditioning regimens are needed to ensure sustained engraftment of the transplanted hematopoietic cells. However, the available data do not allow for recommending specific myeloablative regimen(s).
Timely referral is critical because donor search, donor workup, and procurement of graft typically takes 6 to 12 weeks depending on donor source (ie, longer times are needed for adult unrelated donors). Another, often overlooked, aspect of transplantation associated with survival and graft failure is donor selection. An unaffected HLA-matched sibling, when available, is the “gold standard.” Selecting unrelated adult donors who are HLA-matched to their recipient at the allele level at HLA-A, -B, -C, and -DRB1 results in the best survival and lowest rate of graft failure for nonmalignant diseases.7 A similar approach should be considered when selecting umbilical cord blood units; better matched units are associated with lower rates of graft failure.8 Mismatched related donor HCT, until recently, has been associated with graft manipulation (T-cell depletion) to overcome the HLA barrier. The relatively new approach of using posttransplant cyclophosphamide with a T-cell–replete graft may be an acceptable alternative. However, one must be cautious in adopting strategies that are tested for marrow failure for primary immunodeficiency diseases.9,10
Although it is tempting to recommend phase 2 trials to study optimal transplant conditioning regimens, it is not feasible for very rare diseases. Thus, these studies rely on observational reports and engagement of international collaborations.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal