To the editor:
Antibodies blocking the programmed death 1 receptor (PD-1) on T cells produce tumor regression in multiple cancers by disrupting the PD-L1/PD-1 (programmed death-ligand 1/programmed cell death protein 1) immune inhibitory axis.1-5 This approach to cancer immunotherapy would seem to be an ideal partner for chimeric antigen receptor (CAR)–modified T-cell therapies but is, as yet, untested in this setting. We report a case in which a PD-1 blocking antibody was administered to a patient with refractory diffuse large B-cell lymphoma (DLBCL) and progressive lymphoma after therapy with CAR-modified T cells directed against CD19 (CART19). Following PD-1 blockade, the patient had a clinically significant antitumor response, an expansion of CART19 cells, and decreased coexpression of PD-1 and Eomes by CART19 cells. This case suggests that PD-1 blockade can be effective against cancers failing to respond to CAR-modified T-cell therapy. It also suggests that the PD-1 pathway may be critical in determining the response to CAR-modified T-cell immunotherapy.
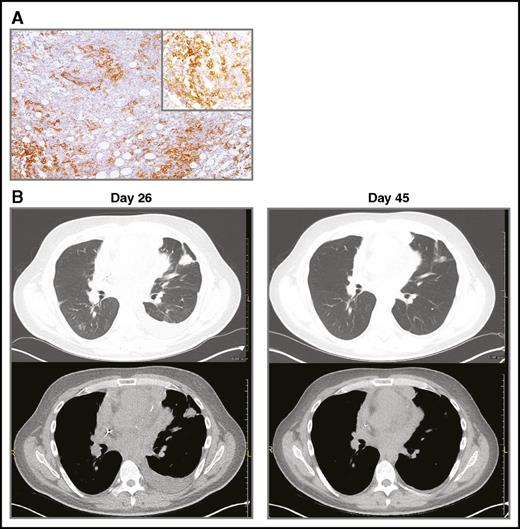
A 35-year-old man with multiply pretreated, refractory DLBCL of primary mediastinal origin with extranodal involvement of small intestine at diagnosis, and mediastinum, lung, myocardium, and pericardium at progression was treated on a clinical trial at the University of Pennsylvania with autologous CART19 cells expressing murine anti-CD19 scFv and 4-1BB-CD3ζ costimulatory-activation domains (NCT02030834).6 CART19 cells were manufactured as previously reported.7,8 He received lymphodepleting chemotherapy with hyperfractionated cyclophosphamide (300 mg/m2 × 6 doses), followed by autologous CART19 cell infusion (5 × 108 CART19 cells or 5.34 × 106 cells/kg), day 0. Follow-up chest computed tomographic (CT) scan performed on day 26 to evaluate worsening dyspnea showed progressive lymphoma with enlargement of mediastinal and pericardial tumor as well as new and enlarging pulmonary nodules. Cardiac magnetic resonance imaging documented new myocardial and pericardial invasion. In view of the patient’s clinical status with rapidly progressive hypoxia and respiratory distress, we did not perform mediastinoscopy or thorascopic lung biopsy at the time of progression. Thus, it was not possible to definitively exclude pseudoprogression as the cause of mediastinal lymph node and pulmonary parenchymal lesions enlargement following CART19. He received pembrolizumab, 2 mg/kg, on day 26 after CART19 cell infusion. Pembrolizumab was chosen for therapy because of preclinical data indicating that anti-PD-1 therapy potently enhances the eradication of established tumors by gene-modified T cells9 and because the patient’s tumor cells strongly expressed PD-L1 (Figure 1A). Other than fever, therapy was well tolerated. By day 45, significant clinical improvement was noted; chest CT scan at that time showed interval improvement of multiple pulmonary nodules, pleural effusion, mediastinal lymphadenopathy, and pericardial nodularity (Figure 1B). Thus, pseudoprogression after CART19 was considered unlikely because there was reduction in the size of lesions after administration of pembrolizumab, rather than further progression. By 3 weeks after therapy, he was able to return to work. Pembrolizumab, 2 mg/kg, was continued every 3 weeks; [18F]-fluorodeoxyglucose positron emission tomography/CT scans on day 67 and day 186 showed continued anatomic improvement in mediastinal adenopathy with residual [18F]-fluorodeoxyglucose uptake (partial metabolic response); pulmonary involvement by lymphoma had resolved. Twelve months after initiation of pembrolizumab, the patient continues to be clinically well.
PD-L1 immunhistochemistry and CT scans demonstrating clinical response to therapy. (A) PD-L1 (CD274) expression by the patient’s DLBCL cells. Biopsy was obtained prior to CART19 cell infusion. Immunohistochemical staining with an anti-PD-L1 antibody from Cell Signaling (clone E1J2J, catalog number 15165BF). The main image is at ×40 magnification; the upper-right corner inset at ×100. Microscope: Leica DM 2500, lens ×10. Camera: Leica, MC 170 HD. Acquisition software: LAS V4.8. (B) CT imaging on day of pembrolizumab infusion (day 26) and 3 weeks after pembrolizumab infusion (day 45).
PD-L1 immunhistochemistry and CT scans demonstrating clinical response to therapy. (A) PD-L1 (CD274) expression by the patient’s DLBCL cells. Biopsy was obtained prior to CART19 cell infusion. Immunohistochemical staining with an anti-PD-L1 antibody from Cell Signaling (clone E1J2J, catalog number 15165BF). The main image is at ×40 magnification; the upper-right corner inset at ×100. Microscope: Leica DM 2500, lens ×10. Camera: Leica, MC 170 HD. Acquisition software: LAS V4.8. (B) CT imaging on day of pembrolizumab infusion (day 26) and 3 weeks after pembrolizumab infusion (day 45).
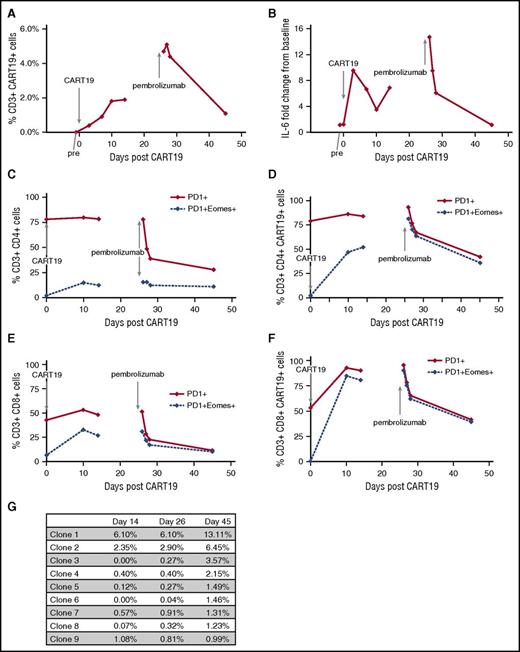
We examined peripheral blood for changes in CART19 DNA by quantitative polymerase chain reaction (data not shown), percentage CART19 cells by flow cytometry, and changes in serum cytokines, including interleukin-6 (IL-6; Figure 2A-B).7 CART19 DNA copy number increased to a maximum of 2350 copies per microgram DNA following CART19 cell infusion and increased again from 497 copies per microgram on day 14 following CART19 (before pembrolizumab) to 1530 copies per microgram on day 26 after pembrolizumab. The percentage of CAR19-expressing T cells increased after CART19 infusion, stabilizing around days 10 to 14; however, for 48 hours after pembrolizumab, we observed the highest percentages CAR19+ T cells (Figure 2A). This increase in percentage of CAR19-expressing T cells reflects an increase in both CAR19+CD8+ and CD4+ T cells after pembrolizumab, particularly the CAR19+CD8+ cells (data not shown). The highest serum IL-6 levels were observed days 3 to 7 following CART19 cell infusion and during the 24 hours after pembrolizumab (Figure 2B). After pembrolizumab infusion, CART19 cells coexpressing PD1/Eomes decreased, especially in the CD8+CAR19+ cells (Figure 2C-F); no changes were observed in cells coexpressing PD-1 and CTLA4, TIM3, or LAG3 (data not shown). Granzyme B+ expression increased after pembrolizumab in both CD4+ and CD8+ T-cell subsets, particularly in CAR19+CD8+ cells (data not shown).
Correlative studies examining changes in T-cell subsets and T-cell clones in relation to CART19 cell infusion and pembrolizumab infusion. (A) Percentage of CART19+CD3+ cells in peripheral blood. Percentage of CART19+ of CD3+ cells prior to CART19 infusion (pre), 3 days after CART19 infusion (day 3), 7 days after CART19 (day 7), 10 days after CART19 (day 10), 14 days after CART19 (day 14), 26 days after CART19 and 1 hour after pembrolizumab infusion (day 26), 27 days after CART19 and 1 day after pembrolizumab (day 27), 28 days after CART19 and 2 days after pembrolizumab (day 28), and 45 days after CART19 and 14 days after pembrolizumab (day 45). (B) Fold change from baseline in IL-6 serum levels. (C) Percentage of PD1+CD4+ cells and PD1+Eomes+CD4+ cells in peripheral blood. (D) Percentage of PD1+CD4+CART19+ cells and PD1+Eomes+CD4+CART19+ cells in peripheral blood. (E) Percentage of PD1+CD8+ cells and PD1+Eomes+C8+ cells in peripheral blood. (F) Percentage of PD1+CD8+CART19+ cells and PD1+Eomes+CD8+CART19+ cells in peripheral blood. (G) Changes in T-cell clones as determined by TCRβ deep sequencing (Adaptive Biotechnologies, immunoSEQ).
Correlative studies examining changes in T-cell subsets and T-cell clones in relation to CART19 cell infusion and pembrolizumab infusion. (A) Percentage of CART19+CD3+ cells in peripheral blood. Percentage of CART19+ of CD3+ cells prior to CART19 infusion (pre), 3 days after CART19 infusion (day 3), 7 days after CART19 (day 7), 10 days after CART19 (day 10), 14 days after CART19 (day 14), 26 days after CART19 and 1 hour after pembrolizumab infusion (day 26), 27 days after CART19 and 1 day after pembrolizumab (day 27), 28 days after CART19 and 2 days after pembrolizumab (day 28), and 45 days after CART19 and 14 days after pembrolizumab (day 45). (B) Fold change from baseline in IL-6 serum levels. (C) Percentage of PD1+CD4+ cells and PD1+Eomes+CD4+ cells in peripheral blood. (D) Percentage of PD1+CD4+CART19+ cells and PD1+Eomes+CD4+CART19+ cells in peripheral blood. (E) Percentage of PD1+CD8+ cells and PD1+Eomes+C8+ cells in peripheral blood. (F) Percentage of PD1+CD8+CART19+ cells and PD1+Eomes+CD8+CART19+ cells in peripheral blood. (G) Changes in T-cell clones as determined by TCRβ deep sequencing (Adaptive Biotechnologies, immunoSEQ).
We performed T-cell receptor β (TCRβ) deep sequencing on the apheresis product, the CAR19-transduced T-cell product, and peripheral blood at day 14 (prior to pembrolizumab), at day 26 (1 hour after pembrolizumab), and at day 45 (19 days after pembrolizumab) (Figure 2G). After pembrolizumab (day 45), we observed 8 dominant clones (frequency ≥1%, range 1.2% to 13.1%). Two of these clones (clones 1 and 2) initially expanded after CART19 cell infusion (day 14) and continued to further expand after pembrolizumab (days 26 to 45). Four clones were present at low levels after CART19 cell infusion and expanded after pembrolizumab (clones 3, 4, 5, and 6). Two dominant clones were only present after pembrolizumab (clones 7 and 8).
Together, our clinical observation and correlative laboratory findings suggest that pembrolizumab may enhance the efficacy of CART19 cells, although the possibility remains that pembrolizumab had activity independent of CART19 cells. This case also suggests a potentially critical role for the PD-1/PD-L1 pathway in CAR-modified T-cell immunotherapy in general. Based on these data, we are currently conducting a phase 1/2 clinical trial of pembrolizumab in patients with CD19+ lymphomas failing to respond to CART19 therapy (NCT02650999).
Authorship
Acknowledgments: The authors acknowledge philanthropic support from James and Frances Maguire, Margarita Louis-Dreyfus, and Sharon Berman for the Lymphoma Program at the Abramson Cancer Center of the University of Pennsylvania.
Contribution: E.A.C. provided clinical data and clinical care for the patient, interpreted the data, and wrote the manuscript; J.J.M. and S.F.L. performed correlative laboratory studies, assisted with data analysis, and edited the manuscript; D.E.A. performed correlative laboratory studies and assisted with data analysis; V.G. assisted with data analysis; B.L.L. directed the CART19 manufacturing program and edited the manuscript; C.H.J. directed the CART19 clinical and research programs and edited the manuscript; S.J.S. is principal investigator for NCT02030834, provided clinical data and clinical care for the patient, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: S.F.L. and S.J.S. receive research support from Novartis. J.J.M., B.L.L., and C.H.J. receive research support from Novartis and have intellectual property rights that have been licensed by the University of Pennsylvania to Novartis. The remaining authors declare no competing financial interests.
Correspondence: Stephen J. Schuster, University of Pennsylvania Perelman Center for Advanced Medicine, 3400 Civic Center Blvd, PCAM 12 South, 157, Philadelphia, PA, 19104; e-mail: stephen.schuster@uphs.upenn.edu.