Key Points
Increased immune suppressors and PD-1 abrogates effector responses in CML patients at diagnosis.
Enhanced net effector immune responses and decreased PD-1 and immune suppressors may promote sustained deep molecular response in CML.
Abstract
Immunological control may contribute to achievement of deep molecular response in chronic myeloid leukemia (CML) patients on tyrosine kinase inhibitor (TKI) therapy and may promote treatment-free remission (TFR). We investigated effector and suppressor immune responses in CML patients at diagnosis (n = 21), on TKI (imatinib, nilotinib, dasatinib) before achieving major molecular response (pre-MMR, BCR-ABL1 >0.1%, n = 8), MMR (BCR-ABL1 ≤0.1%, n = 20), molecular response4.5 (MR4.5, BCR-ABL1 ≤0.0032%, n = 16), and sustained TFR (BCR-ABL1 undetectable following cessation of TKI therapy, n = 13). Aberrant immune-inhibitory responses (myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and programmed death-1 (PD-1) inhibitory molecule expression on CD4+/CD8+ T cells were increased in CML patients at diagnosis. Consequent quantitative and functional defects of innate effector natural killer (NK) cells and cytotoxic T-lymphocyte responses to leukemia-associated antigens WT1, BMI-1, PR3, and PRAME were observed at diagnosis. Treg and PD-1+CD4+/CD8+ T cells persisted in pre-MMR CML patients on TKI. Patients in MMR and MR4.5 had a more mature, cytolytic CD57+CD62L− NK cell phenotype, consistent with restoration of NK cell activating and inhibitory receptor repertoire to normal healthy donor levels. Immune responses were retained in TFR patients off-therapy, suggesting the restored immune control observed in MMR and MR4.5 is not an entirely TKI-mediated effect. Maximal restoration of immune responses occurred only in MR4.5, as demonstrated by increased NK cell and effector T-cell cytolytic function, reduced T-cell PD-1 expression and reduced numbers of monocytic MDSCs.
Introduction
Tyrosine kinase inhibitors (TKIs) targeting Bcr-Abl represent the current standard of care for patients with chronic myeloid leukemia (CML) in chronic phase (CP). Recently, the achievement of deep molecular responses (DMRs) with TKIs has afforded select patients the possibility to discontinue therapy without relapse.1,2 A number of phenotypic and functional aberrations of the immune system have been described in CML patients at diagnosis, which tend to worsen with disease progression.3,4 In contrast, TKI-mediated immunomodulatory effects conferring immune system reactivation have also been reported in CML and gastrointestinal stromal tumor patients.5 However, less is known regarding the potential contributory role of the immune system in patients achieving a sustained DMR to TKI therapy in CML.
Immune-inhibitory mechanisms predominate within the leukemic microenvironment in newly diagnosed CML patients, linked to an expansion of immunosuppressive myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), which function as suppressors of host anti-CML immune responses.6 In the context of immune effector responses, natural killer (NK) cell immunodeficiency in CP-CML patients at diagnosis has been reported previously,7,8 whereas early disease relapse following TKI discontinuation in CML is associated with both a low number and impaired function of NK cells.9,10 In addition, CML-specific cytotoxic T lymphocytes (CTLs), primed to recognize aberrant antigens on leukemic cells, upregulate the costimulatory/inhibitory receptor programmed death-1 (PD-1), expressed on activated T cells, promoting an exhausted T-cell phenotype and impaired T-cell function.11
We studied effector and suppressor immune responses over time in CP-CML patients at diagnosis and on TKIs before achievement of major molecular response (pre-MMR, BCR-ABL1 >0.1%), at MMR (BCR-ABL1 ≤0.1%) and molecular response4.5 (MR4.5, BCR-ABL1 ≤0.0032%), and in CML patients in sustained treatment-free remission (TFR; BCR-ABL1 undetectable following cessation of TKI therapy). Sustained DMR (≥4-log reduction in BCR-ABL1 transcript levels) is a current eligibility requirement for most TFR trials1 ; therefore, this study may help identify potential immunological prognostic markers that can be investigated in future TKI discontinuation studies. We found immune effector responses of NK cells, including maturation status and activating/inhibitory receptor repertoire, and functional CTL immune responses against several leukemia-associated antigens (LAAs) were downregulated in CML patients at diagnosis. In contrast, these immune effector responses were restored to normal levels in CML patients who achieved MMR and MR4.5 on TKIs and were retained in TFR patients who achieved a durable DMR on imatinib. However, compared with MMR, NK cell cytolytic effector function was restored to normal levels only in MR4.5. Enhanced immune effector responses were associated with a concomitant reduction to normal levels in both immune suppressor monocytic (Mo)-MDSC activity and PD-1 expression on effector CD4+ and CD8+ T cells only in MR4.5 patients. Enhanced net effector immune responses and decreased PD-1 and immune suppressors may promote sustained DMR in CML.
Methodology
Patient samples
Peripheral blood (PB) samples were obtained from 78 CP-CML patients at diagnosis (n = 21), pre-MMR (n = 8; median, 12.5 months [range, 3-31] of TKI therapy), MMR (n = 20), MR4.5 (n = 16), or in stable TFR (n = 13; CML 8 TWISTER trial; median TFR duration, 6 months [range, 3-22 months]; median study follow-up, 66 months), and healthy blood donors (n = 7). Multiple samples were collected for some patients; therefore, 105 samples were used in this study (n = 21 at diagnosis, n = 14 pre-MMR, n = 27 MMR, n = 23 MR4.5, and n = 20 TFR) (Table 1). Eight patients were serially followed for data at multiple time points. Pre-MMR represents the period before MMR is achieved, with BCR-ABL1 transcript levels >0.1% and <10%. Pre-MMR, MMR, and MR4.5 cohorts contained a similar percentage of patients treated with imatinib, nilotinib, and dasatinib. No lymphocytosis associated with the proliferation of clonal CTLs or NK cells12 was observed in dasatinib treated patients. The study was approved by the institutional Human Research Ethics Committee and conducted in accordance with the Declaration of Helsinki. PB mononucleated cells (PBMCs) from patients or healthy donors were isolated by Lymphoprep (STEMCELL Technologies, Vancouver, BC, Canada) density gradient centrifugation and cryopreserved until use. BCR-ABL1 levels were standardized to the international standard and expressed as a percentage.
Patient characteristics
| . | Diagnosis CP-CML . | Pre-MMR . | MMR . | MR4.5 . | TFR . |
|---|---|---|---|---|---|
| BCR-ABL1 | ≥10% | >0.1%, <10% | ≤0.1%, >0.0032% | ≤0.0032%* | 0% |
| Patients, n | 21 | 8 | 20 | 16 | 13 |
| Age at diagnosis, median (range), y | 52 (34-85) | NA | NA | NA | NA |
| Sex, % | |||||
| Male | 52 | 62 | 67 | 56 | 46 |
| Female | 48 | 38 | 33 | 44 | 54 |
| TKI duration, median (range), mo | 0 | 12.5 (3-31) | 36 (5-132) | 31 (8-150) | NA† |
| TKI therapy, % | |||||
| Imatinib | NA | 50 | 48 | 65 | NA |
| Nilotinib | NA | 38 | 37 | 22 | NA |
| Dasatinib | NA | 12 | 15 | 13 | NA |
| . | Diagnosis CP-CML . | Pre-MMR . | MMR . | MR4.5 . | TFR . |
|---|---|---|---|---|---|
| BCR-ABL1 | ≥10% | >0.1%, <10% | ≤0.1%, >0.0032% | ≤0.0032%* | 0% |
| Patients, n | 21 | 8 | 20 | 16 | 13 |
| Age at diagnosis, median (range), y | 52 (34-85) | NA | NA | NA | NA |
| Sex, % | |||||
| Male | 52 | 62 | 67 | 56 | 46 |
| Female | 48 | 38 | 33 | 44 | 54 |
| TKI duration, median (range), mo | 0 | 12.5 (3-31) | 36 (5-132) | 31 (8-150) | NA† |
| TKI therapy, % | |||||
| Imatinib | NA | 50 | 48 | 65 | NA |
| Nilotinib | NA | 38 | 37 | 22 | NA |
| Dasatinib | NA | 12 | 15 | 13 | NA |
NA = not applicable.
15/16 (93.7%) patients had undetectable BCR-ABL1 transcripts at time of sample collection.
TFR patients have been off TKIs for a median of 6 months (range, 3-22 months).
Monoclonal antibodies and flow cytometric analysis
The following antibodies were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), or Beckman Coulter (Fullerton, CA), and used for phenotypic analysis of freshly thawed PBMC. NK cells: fluorescein isothiocyanate (FITC)-CD161 and CD94, phycoerythrin (PE)-CD337 (NKp30), CD159a (NKG2A) and CD1d, PE Texas Red-CD16, PerCP eFluor710-CD336 (NKp44), PerCP-CD159c (NKG2C), PerCP-Cy5.5-CD8, PeCy7-CD62L and CD314 (NKG2D), PC7-CD158b1/b2/j (killer-immunoglobulin like receptor 2 [KIR2]DL2/DL3/DS2), APC-CD335 (NKp46) and CD158f (KIR2DL5), AlexaFluor700-CD56, APC eFluor780-CD3, APCCy7-CD69, eFluor450-CD57, and Krome Orange-CD3. MDSCs: FITC-CD3, CD16, CD19 and CD56, PE-CD66b, PerCP-Cy5.5-CD15, PeCy7-CD14, APC-HLA-DR, APCCy7-CD11b, and V450-CD33. Tregs: FITC-CD4, PE-CD25, PerCP-Cy5.5-CD3, PeCy7-CD45RO, APC-FoxP3, APC eFluor780-CD27, and V450-CD127. PD-1: FITC-CD3, PE-CD279, PE Texas Red-CD16, PerCP-CD19, PeCy7-CD14, APC-CD8, AlexaFluor700-CD56, and BV421-CD20. B cells: FITC-immunoglobulin D, PE-CD24, ECD-CD38, PerCP-Cy5.5-CD3, APC-immunoglobulin M, APC-AlexaFluor700-CD19, APC eFluor780-CD27, and BV421-CD20.
Live dead aqua or Live dead far red (Invitrogen, Carlsbad, CA) fixable dead cell stain was used to assess viability. For surface staining, cells were incubated with titrated antibody for 30 minutes on ice. Intracellular FoxP3 staining (FoxP3/transcription factor staining buffer set, eBioscience) was performed according to the manufacturer’s recommendations. Cells were acquired with the BD LSRFortessa X-20 (BD Biosciences) and data analyzed with FlowJo v10.1 (FlowJo, LLC, Ashland, OR). Unless otherwise noted, cell populations of interest are reported as a proportion of total lymphocytes, derived from side scatter vs forward scatter (FSC) gated lymphocytes, with doublet exclusion (FSC-A vs FSC-H) and dead cell discrimination (dead cell stain−). Fluorescence minus 1 controls were used to identify gating boundaries. The absolute number of cells was calculated as follows [total lymphocyte count (cells/μL) × percent cells]/100.
CD107a degranulation assay
To measure NK cell cytotoxicity, CD107a degranulation was used with modifications.13 Briefly, 5 × 105 PBMCs were cocultured with 5 × 104 K562 target cells for 4 hours at 37°C with FITC-CD107a (clone H4A3, BD Biosciences) and the protein transport inhibitor monensin (BD GolgiStop). Cells were subsequently stained with CD3-APC eFluor780 and CD56-AlexaFluor700 (BD Biosciences) to determine the percentage of CD107a+ NK cells by flow cytometry. Live dead far red fixable dead cell stain was included in each tube. Phorbol 12-myristate 13-acetate (50 ng/mL) plus ionomycin (500 ng/mL) (Sigma Aldrich, St Louis, MO) were used as positive control of degranulation. Negative controls received RF10 culture medium (RPMI containing 10% fetal calf serum, Sigma Aldrich).
Peptide synthesis
Peptide libraries for preferentially expressed antigen in melanoma (PRAME), Wilms’ tumor 1 (WT1), B cell–specific Moloney murine leukemia virus integration site 1 (BMI-1) and proteinase 3 (PR3) of 15-mer peptides overlapping by 11 amino acids spanning the entire protein were manufactured by Biosynthesis (Lewisville, TX) to a minimum purity of 95%. Control pool pepmix for human cytomegalovirus A pp65 and negative control pool pepmix for HIV-1 were obtained from JPT Peptide Technologies (Berlin, Germany). HLA-A*0201–restricted peptides for PRAME; VLDGLDVLL100-108, SLYSFPEPEA142-151, ALYVDSLFFL300-309, SLLQHLIGL425-433, NLTHVLYPV435-443, WT1; VLDFAPPGA37-45, RMFPNAPYL126-134, SLGEQQYSV187-195, CMTWNQMNL235-243, BMI-1; CLPSPSTPV271-279, TLQDIVYKL74-82, PR-1; VLQELNVTV169-177, HIV; SLYNTVATLY61-70 and CMVpp65; and NLVPMVATV495-503 were also obtained from Biosynthesis.
ELISPOT assay
The frequency of antigen-specific CTLs in PBMCs from patients or healthy donors expanded for 7 days in vitro was assessed in a modified enzyme-linked immunospot (ELISPOT) assay.14-16 A total of 1 × 105 PBMCs were plated in 96-well round-bottom plates and loaded with peptide libraries at 0.1 or 0.35 μg/mL or individually with single peptides in HLA-A*0201+ patients at 1 μg/mL. Results from peptide libraries and single peptides in individual patients were combined for analysis. Lyophilized peptides were dissolved in dimethyl sulfoxide and diluted in assay medium for individual experiments. Freshly prepared dimethyl sulfoxide vehicle control was used as a negative control for all experiments. Staphylococcal enterotoxin B (2 μg/mL) was used as a positive control. Antigen-specific CTL response was enhanced by supplementing the culture media with interleukin-12 (2 ng/mL) and interleukin-2 (1 ng/mL). Cells were harvested on day 7 for detection of interferon-γ (IFN-γ) by ELISPOT analysis using AID iSpot Flurospot Reader System (Strassberg, Germany). A positive ELISPOT response was defined as at least 20 spots per 1 × 105 PBMC and significant by t test (P ≤ .05) in the presence of viable negative and positive controls.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v6 software (GraphPad Software Inc, La Jolla, CA). Unpaired Student t test (Mann-Whitney test) was used for comparing differences between groups. Data are given as mean ± standard error mean. A P value of ≤.05 was considered significant.
Results
CML patients in MMR and MR4.5 have increased NK cell numbers displaying a more mature NK cell phenotype compared with diagnosis
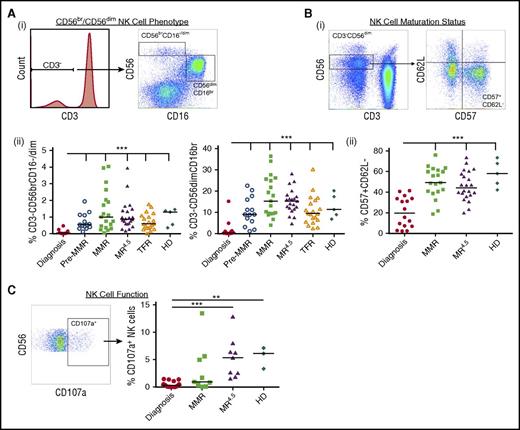
CD56bright (br; immunoregulatory) NK cells as a percentage of total lymphocytes were significantly decreased in CML patients at diagnosis (0.07 ± 0.03%), compared with pre-MMR (0.70 ± 0.13%), MMR (1.4 ± 0.3%), MR4.5 (1.1 ± 0.2%), and TFR (0.7 ± 0.1%) (all P < .0001). Similarly, CD56dim (cytolytic) NK cells were significantly decreased in CML patients at diagnosis (1.6 ± 0.9%), compared with pre-MMR (10.4 ± 1.8%), MMR (17.4 ± 2.2%), MR4.5 (15.3 ± 1.2%), and TFR (11.0 ± 1.5%) (all P < .0001, Figure 1Aii).
NK cell phenotype and function in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in healthy donors (HDs). (A) (i) CD3−CD56brCD16−/dim (immunoregulatory) and CD3−CD56dimCD16br (cytolytic) NK cell gating. (ii) Percent expression CD3−CD56brCD16−/dim and CD3−CD56dimCD16br NK cells as a proportion of total lymphocytes. (B) (i) CD57+CD62L− NK cell gating. (ii) Percent expression CD57+CD62L− (of total NK cells). (C) Representative dot plot of CD107a+ expression in CD3−CD56dim NK cells (left) and percent CD107a+ NK cells in CML patients (of total lymphocytes). Bars denote the median. ***P ≤ .001; **P ≤ .01.
NK cell phenotype and function in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in healthy donors (HDs). (A) (i) CD3−CD56brCD16−/dim (immunoregulatory) and CD3−CD56dimCD16br (cytolytic) NK cell gating. (ii) Percent expression CD3−CD56brCD16−/dim and CD3−CD56dimCD16br NK cells as a proportion of total lymphocytes. (B) (i) CD57+CD62L− NK cell gating. (ii) Percent expression CD57+CD62L− (of total NK cells). (C) Representative dot plot of CD107a+ expression in CD3−CD56dim NK cells (left) and percent CD107a+ NK cells in CML patients (of total lymphocytes). Bars denote the median. ***P ≤ .001; **P ≤ .01.
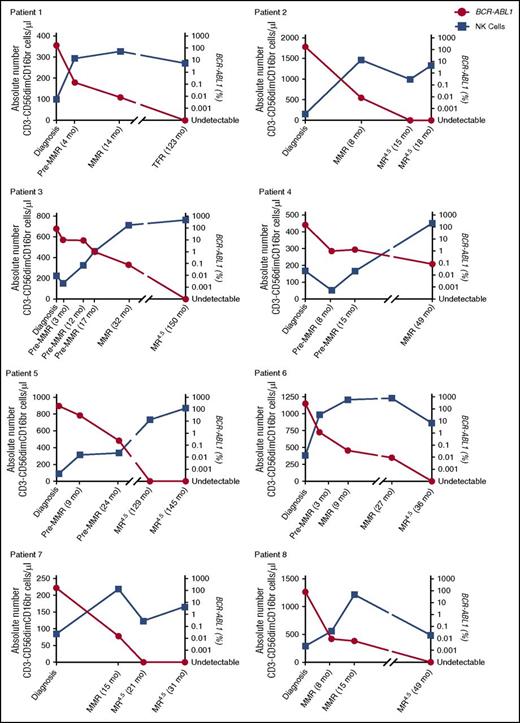
More detailed phenotypic and functional characterizations of CD3−CD56dim NK cells were performed on CML patients at diagnosis, MMR, and MR4.5 and in healthy donors. CD57+CD62L− coexpression, indicative of a more mature NK cell phenotype, was increased in MMR (49.6 ± 3.5%) and MR4.5 (46.2 ± 3.0%), compared with diagnosis (20.7% ± 3.7%) (both P < .0001, Figure 1Bii). We investigated NK cell CD107a degranulation as a surrogate marker of NK cell function and observed decreased CD107a+ expression in CML patients at diagnosis (0.5 ± 0.1%), which was only restored to normal levels in MR4.5 (5.3 ± 1.2%, P < .0001, Figure 1C). The absolute number of CD3−CD56dimCD16br NK cells/μL was lowest in CML patients at diagnosis when BCR-ABL1 transcript levels were higher. In the majority of patients, a maximal increase in the absolute number of NK cells occurred in MMR; however, NK cell number was still increased in all patients in MR4.5 compared with diagnosis (Figure 2, n = 8 individual patients assessed serially).
Serial analyses of absolute numbers of NK cells per microliter PB in CML patients at diagnosis, pre-MMR, MMR, MR4.5, and TFR. Patients 1 through 8 showing the absolute number of CD3−CD56dimCD16br cells per microliter (blue) against BCR-ABL1 (red) over time. Duration of treatment with TKI is indicated in months (mo) from diagnosis.
Serial analyses of absolute numbers of NK cells per microliter PB in CML patients at diagnosis, pre-MMR, MMR, MR4.5, and TFR. Patients 1 through 8 showing the absolute number of CD3−CD56dimCD16br cells per microliter (blue) against BCR-ABL1 (red) over time. Duration of treatment with TKI is indicated in months (mo) from diagnosis.
Expression of NK cell–activating receptors CD161, CD94/NKG2C, and NKG2D, natural cytotoxicity receptors NKp30 and NKp46 and KIRs KIR2DL2/DL3/DS2 was restored to normal levels in MMR and MR4.5 compared with their downregulation at diagnosis (see supplemental Figure 1, available on the Blood Web site). No significant difference was observed in effector CD3+CD56+ NK-like T-cell expression between the CML patient groups or healthy donors (supplemental Figure 2). Expression of CD1d, a critical antigen-presenting molecule involved in the activation of NK-like T cells, was decreased on monocytes in CML patients at diagnosis (30 ± 6.5%) and restored to normal levels in MMR (64.6 ± 5.5%) and MR4.5 (68.7 ± 5.0%) (both P < .0001), suggesting the antigen-presenting function of monocytes may be deficient in CML patients at diagnosis.
CML patients in MMR and MR4.5 have increased effector CTL immune responses to LAAs compared with diagnosis
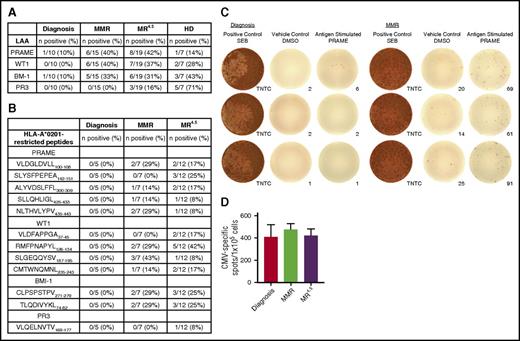
A blunted antigen-specific CTL response to LAAs PRAME, WT1, BMI-1, and PR3 was observed in CML patients at diagnosis (Figure 3A-C); however, when the leukemic cell load was lower in MMR and MR4.5, there was a marked increase in effector T-cell response to LAAs. PRAME and BMI-1 responses were detected in 10% of CML patients at diagnosis; no response was detected for WT1 or PR3. In contrast, PRAME and WT1 were the most abundant CTL responses detected in MMR (PRAME, 40%; WT1, 40%) and MR4.5 (PRAME, 42%; WT1, 37%). BMI-1 responses were detected in 33% and 31% of patients in MMR and MR4.5, respectively. No detectable PR3-CTL response was observed in MMR and a low (16%) PR3-CTL response was observed in MR4.5. CMV-CTL responses did not differ between CMV-positive patients at diagnosis, MMR, or MR4.5 (Figure 3D), suggesting the enhanced CTL response to LAAs observed in vitro are not due to an overall increase in CTL responses resulting from a CMV reactivation.
T-cell responses to LAAs PRAME, WT1, BMI-1, and PR3 in CML patients at diagnosis, MMR, MR4.5, and in HD. (A) CTL-mediated immune responses to LAAs in CML patients at diagnosis (n = 10), MMR (n = 15), and MR4.5 (n = 19) and in HD (n = 7). (B) CTL-mediated immune responses to HLA-A*0201–restricted peptides in CML patients at diagnosis (n = 5), MMR (n = 7), and MR4.5 (n = 12). (C) Representative IFN-γ ELISPOT showing T-cell response to PRAME in the same patient at diagnosis (no response, left) and MMR (positive response with IFN-γ production, right). Each colored spot represents a single IFN-γ–secreting cell. SEB, staphylococcal enterotoxin B; TNTC, too numerous to count. Images were captured and IFN-γ spot counts were assessed using AID iSpot Flurospot Reader System. (D) CTL-mediated immune responses to CMV in CMV-positive patients at diagnosis (n = 4), MMR (n = 6), and MR4.5 (n = 10). Columns represent the mean number of CMV-specific spots/1 × 105 cells.
T-cell responses to LAAs PRAME, WT1, BMI-1, and PR3 in CML patients at diagnosis, MMR, MR4.5, and in HD. (A) CTL-mediated immune responses to LAAs in CML patients at diagnosis (n = 10), MMR (n = 15), and MR4.5 (n = 19) and in HD (n = 7). (B) CTL-mediated immune responses to HLA-A*0201–restricted peptides in CML patients at diagnosis (n = 5), MMR (n = 7), and MR4.5 (n = 12). (C) Representative IFN-γ ELISPOT showing T-cell response to PRAME in the same patient at diagnosis (no response, left) and MMR (positive response with IFN-γ production, right). Each colored spot represents a single IFN-γ–secreting cell. SEB, staphylococcal enterotoxin B; TNTC, too numerous to count. Images were captured and IFN-γ spot counts were assessed using AID iSpot Flurospot Reader System. (D) CTL-mediated immune responses to CMV in CMV-positive patients at diagnosis (n = 4), MMR (n = 6), and MR4.5 (n = 10). Columns represent the mean number of CMV-specific spots/1 × 105 cells.
CML patients in MMR and MR4.5 have reduced immune suppressor MDSCs and Tregs compared with diagnosis
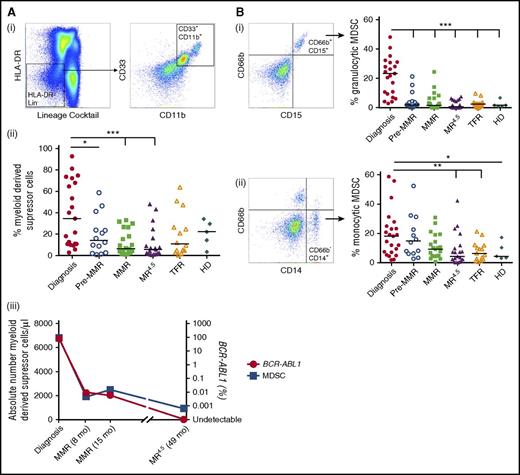
The percentage of HLA-DR−Lineage (LIN)−CD11b+CD33+ MDSC was increased at diagnosis (38.6 ± 6.5%) compared with pre-MMR (19.1 ± 4.9%, P = .04), MMR (11.8 ± 2.5%, P = .0004), MR4.5 (11.4 ± 3.3%, P = .0001), and TFR (19.6 ± 5.5%, P = .03) (Figure 4Aii). In keeping with the expression data, the absolute number of MDSCs per microliter was highest in CML patients at diagnosis when BCR-ABL1 transcript levels were higher, compared with CML patients on TKIs with reduced BCR-ABL1 levels (Figure 4Aiii).
Frequency of MDSCs in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in HD. (A) (i) HLA-DR−LIN−CD11b+CD33+ MDSC gating. (ii) MDSCs as a percentage of PBMCs. (iii) Representative patient (of 8 patient samples) showing the absolute number of MDSCs per microliter (blue) against BCR-ABL1 (red) over time. (B) (i) CD66b+CD15+ Granulocytic (Gr)-MDSC gating (left) and percent Gr-MDSC (right) in CML patients. (ii) CD66b−CD14+ Mo-MDSC gating (left) and percent Mo-MDSC (right) in CML patients. Bars denote the median. MDSCs were derived from side scatter vs FSC gated bulk PBMCs, with doublet exclusion (FSC-A vs FSC-H) and dead cell discrimination (dead cell stain−). ***P ≤ .001; **P ≤ .01; *P ≤ .05.
Frequency of MDSCs in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in HD. (A) (i) HLA-DR−LIN−CD11b+CD33+ MDSC gating. (ii) MDSCs as a percentage of PBMCs. (iii) Representative patient (of 8 patient samples) showing the absolute number of MDSCs per microliter (blue) against BCR-ABL1 (red) over time. (B) (i) CD66b+CD15+ Granulocytic (Gr)-MDSC gating (left) and percent Gr-MDSC (right) in CML patients. (ii) CD66b−CD14+ Mo-MDSC gating (left) and percent Mo-MDSC (right) in CML patients. Bars denote the median. MDSCs were derived from side scatter vs FSC gated bulk PBMCs, with doublet exclusion (FSC-A vs FSC-H) and dead cell discrimination (dead cell stain−). ***P ≤ .001; **P ≤ .01; *P ≤ .05.
Granulocytic MDSCs (HLA-DR−LIN−CD11b+CD33+CD66b+CD15+) were significantly increased in CML patients at diagnosis (22.9 ± 3.2%) compared with pre-MMR (4.9 ± 1.7%), MMR (4.0 ± 1.3%), MR4.5 (2.2 ± 0.6%), and TFR (2.5 ± 0.7%) (all P < .0001). Mo-MDSCs (HLA-DR−LIN−CD11b+CD33+CD66b−CD14+) were also increased at diagnosis (19.4 ± 3.3%) compared with healthy donors (7.7 ± 2.7%, P = .052) and only significantly decreased to normal levels in CML patients in MR4.5 (8.6 ± 2.3%, P = .003) and TFR (7.8 ± 1.7%, P = .007) (Figure 4B).
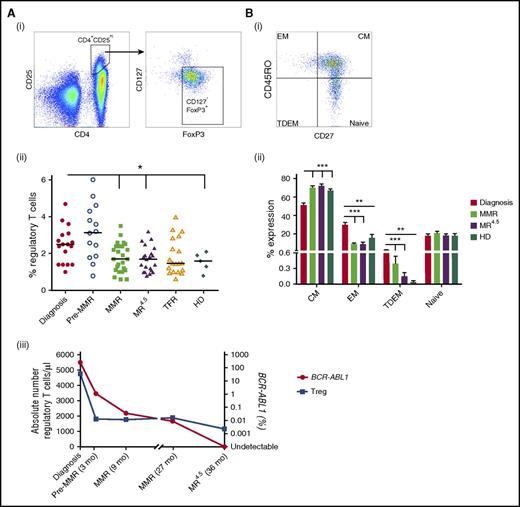
CD4+CD25hiCD127−FoxP3+ Treg percentage was higher in CML patients at diagnosis (2.3 ± 0.2%) and pre-MMR (3.1 ± 0.4%) compared with MMR (1.8 ± 0.2%, P = .02), MR4.5 (1.7 ± 0.1%, P = .01), and TFR (1.8 ± 0.2%) (Figure 5Aii). The absolute number of Tregs per microliter was highest in CML patients at diagnosis when BCR-ABL1 transcript levels were higher compared with CML patients on TKIs with reduced BCR-ABL1 levels (Figure 5Aiii). Further characterization of naïve/memory Treg subsets in CML patients at diagnosis, MMR, and MR4.5 showed an increased effector memory phenotype in CML patients at diagnosis compared with patients in MMR and MR4.5 who displayed a predominantly central memory phenotype (Figure 5B).
Frequency of Tregs in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in HD. (A) (i) CD4+CD25hiCD127−FoxP3+ Treg gating. (ii) Treg percentage as a proportion of CD4+ T cells. (iii) Representative patient (of 8 patient samples) showing the absolute number of Tregs per microliter (blue) against BCR-ABL1 (red) over time. (B) (i) Effector memory (EM), central memory (CM), naïve (N), terminally differentiated effector memory (TDEM) Treg subset gating. (ii) Percent EM, CM, N, and TDEM subsets in CML patients at diagnosis (n = 18), MMR (n = 25), and MR4.5 (n = 22) and in HD (n = 5) as a proportion of CD4+ T cells. Bars denote the median. ***P ≤ .001; **P ≤ .01; *P ≤ .05.
Frequency of Tregs in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in HD. (A) (i) CD4+CD25hiCD127−FoxP3+ Treg gating. (ii) Treg percentage as a proportion of CD4+ T cells. (iii) Representative patient (of 8 patient samples) showing the absolute number of Tregs per microliter (blue) against BCR-ABL1 (red) over time. (B) (i) Effector memory (EM), central memory (CM), naïve (N), terminally differentiated effector memory (TDEM) Treg subset gating. (ii) Percent EM, CM, N, and TDEM subsets in CML patients at diagnosis (n = 18), MMR (n = 25), and MR4.5 (n = 22) and in HD (n = 5) as a proportion of CD4+ T cells. Bars denote the median. ***P ≤ .001; **P ≤ .01; *P ≤ .05.
Increased levels of PD-1 on CD8+ and CD4+ T cells in CML patients at diagnosis are restored to normal levels at MR4.5
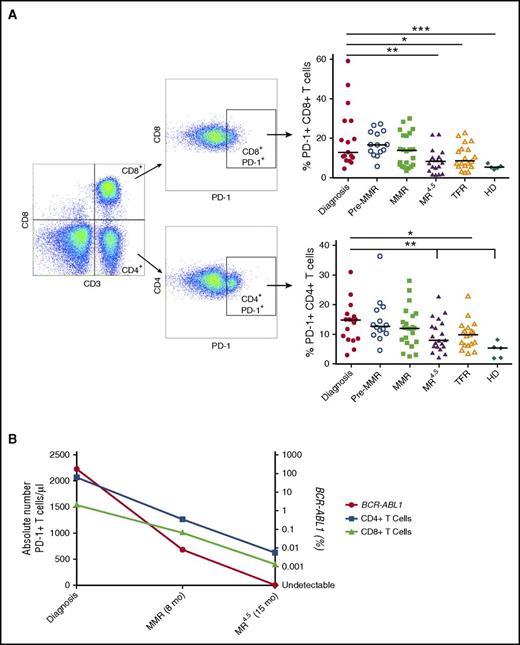
PD-1+CD8+ T-cell expression was increased in CML patients at diagnosis (20.1 ± 3.7%) and pre-MMR (17.1 ± 1.7%) compared with healthy donors (5.5 ± 0.5%, P = .0009), and only significantly reduced to normal levels at MR4.5 (8.4 ± 1.2%, P = .002) and TFR (10.5 ± 1.4%, P = .02). Similarly, PD-1+CD4+ T-cell expression was also increased in CML patients at diagnosis (13.7 ± 1.6%) and pre-MMR (14.5 ± 2.0%) compared with healthy donors (4.6 ± 1.2%, P = .004), and only significantly reduced to normal levels at MR4.5 (9.6 ± 1.2%, P = .004) and TFR (9.8 ± 1.0%, P = .04) (Figure 6A). In keeping with the expression data, the absolute number of PD-1+ T cells per microliter was highest in CML patients at diagnosis when BCR-ABL1 transcript levels were higher compared with CML patients on TKIs with reduced BCR-ABL1 levels (Figure 6B). There was no significant difference in PD-1 expression on NK cells, B cells, or monocytes in CML patients at diagnosis compared with pre-MMR, MMR, MR4.5, and TFR (data not shown).
CD4+and CD8+T-cell PD-1 expression in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in HD. (A) CD3+/CD4+/CD8+ T-cell gating to assess PD-1 expression. CD4+ and CD8+ T-cell PD-1+ expression is shown as a percentage of total lymphocytes. (B) Representative patient (of 8 patient samples) showing the absolute number of CD4+PD-1+ (blue) and CD8+PD-1+ (green) T cells per microliter against BCR-ABL1 (red) over time. Bars denote the median. ***P ≤ .001; **P ≤ .01; *P ≤ .05.
CD4+and CD8+T-cell PD-1 expression in CML patients at diagnosis, pre-MMR, MMR, MR4.5, TFR, and in HD. (A) CD3+/CD4+/CD8+ T-cell gating to assess PD-1 expression. CD4+ and CD8+ T-cell PD-1+ expression is shown as a percentage of total lymphocytes. (B) Representative patient (of 8 patient samples) showing the absolute number of CD4+PD-1+ (blue) and CD8+PD-1+ (green) T cells per microliter against BCR-ABL1 (red) over time. Bars denote the median. ***P ≤ .001; **P ≤ .01; *P ≤ .05.
In addition to PD-1 expression on B cells, we performed extensive characterization of major B-cell subsets including transitional, naïve, nonswitched memory, class-switched memory, plasmablasts, and plasma cells (supplemental Figure 3). Nonswitched memory B-cell expression was decreased in CML patients at diagnosis, MMR, and MR4.5 compared with healthy donors. Loss of memory B-cell subsets in CML patients at diagnosis and following complete cytogenetic remission on imatinib was reported by de Lavallade et al.17 Our study further developed these findings, showing memory B-cell expression fails to normalize despite achievement of DMR on imatinib, nilotinib, or dasatinib.
Discussion
The immune system has long been implicated in the control of CML, as evidenced by the therapeutic efficacy of allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusion-mediated graft-versus-leukemia effects.18 More recently, the goal in CML treatment is to induce a durable DMR as a prelude to successful TFR, with a lack of overt relapse in such patients attributed to immunological control of CML.19 Several studies of combination TKI therapy and IFN-α have been completed recently, with several reporting increased rates of DMR.20-22 A phase 1 trial (NCT02011945) of dasatinib and nivolumab, a PD-1 inhibitor, is recruiting, with MMR and DMR incidence as planned outcome measures. LAAs such as WT1, PRAME, PR3, and BMI-1 in CML patients represent attractive targets for immunotherapy,23-29 with efficacy demonstrated in vaccination, including dendritic cell vaccination,30-33 and, more recently, T-cell receptor–mimic antibodies.34-36 In support of these studies, we observed maximal restoration of immune recovery, as demonstrated by increased effector NK cell and T-cell immune responses, reduced PD-1 inhibitory molecule expression on CD4+ and CD8+ T cells, and reduced numbers of Mo-MDSC in MR4.5 only, suggesting DMR may be the preferred threshold for future TFR studies to benefit from the improved effector immune responses. Therapeutic methods to enhance NK cell function and immunogenic CTL responses early or blocking aberrant PD-1 signaling may result in greater rate of success in TKI cessation studies.
The strength of our study lies in our comprehensive assessment of innate, adaptive, humoral, and suppressor immune responses in CML patients over time. CML patients in MMR and MR4.5 displayed a more mature (CD57+CD62L−) cytolytic phenotype and restored KIR2DL2/DL3/DS2 expression compared with diagnosis, the latter being strongly associated with increasing CD57 acquired in the later stages of NK cell maturation.37 Decreased NKp30 and NKp46 activating receptor expression was observed in CML patients at diagnosis compared with patients in MMR and MR4.5. Downregulation of NKp30 and NKp46 has been reported previously in acute myeloid leukemia and chronic lymphocytic leukemia and shown to correlate with decreased NK cell cytotoxicity.38-40 Reduced NKG2D activating receptor expression has been previously identified in CML patients at diagnosis, promoting leukemic cell survival.41 Our study extends these findings, identifying reduced expression of the NKG2 family of C-type lectin receptors—CD94/NKG2A, CD94/NKG2C, and NKG2D—at diagnosis and confirming that receptor expression was restored to normal levels following achievement of DMR to TKI therapy. Unfortunately, our study did not have sufficient power to detect whether achievement of MMR or MR4.5, with second-generation TKIs dasatinib and nilotinib, which have the capacity to induce more rapid DMR,42 correlates with improved immune function.
To determine the extent to which our observation of progressive immune recovery can be explained by depth of response, rather than an entirely TKI-mediated effect (the latter would be expected to be seen almost immediately following treatment and subsequently lost following TKI discontinuation), we assessed immune responses in CML patients early (pre-MMR) and following successful imatinib discontinuation in TFR. Our TFR cohort included CML patients in stable TFR only and not patients who relapsed following TKI discontinuation because the immune system has been identified as an important factor for maintenance of TFR,43 suggesting the immune system in relapse patients may be compromised and would therefore not represent an appropriate off-TKI comparison group. However, comparison of immune responses in relapse vs nonrelapse TFR patients is the focus of an ongoing study. MDSC levels decreased early, likely as a result of an on-target TKI effect because MDSCs are part of the tumor clone displaying BCR-ABL1 expression.44 Giallongo et al45 have previously shown MDSCs decrease to normal levels in CML patients following imatinib therapy, and the TKI sunitinib has been shown to reduce the frequency of MDSCs and reverse T-cell immune suppression in the PB of metastatic renal cell carcinoma patients.46 MDSCs are present in the PB circulation in healthy individuals; however, they lack suppressive activity.47
Both Treg and CD4+/CD8+ T-cell PD-1 expression was increased in CML patients at diagnosis and pre-MMR compared with healthy donors, suggesting that at least some level of immune suppression remains active early during the course of TKI treatment. Tregs displayed a predominantly effector memory phenotype in CML patients at diagnosis, switching to a central memory phenotype in MMR and MR4.5. Mailloux et al48 have described Treg-memory phenotype switching to be a poor prognostic indicator in myelodysplastic syndrome and isolated effector memory Tregs were shown to be significantly more suppressive than central memory Tregs in vitro. Further research is required to ascertain the functional role effector memory Treg expansion may play in a patient’s ability to achieve sustained DMR on therapy.
In our cohort, both MMR and MR4.5 patients had a similar median duration of TKI treatment (36 and 31 months, respectively). It is therefore possible that the restored immune responses observed in MR4.5 are related to our patient population having a more rapid and deeper response on TKIs. Alternatively, DMR may be facilitated by the more favorable immune response. Iriyama et al49 have shown that increased numbers of NK cells and CTLs are associated with achievement of DMR in dasatinib-treated CP-CML patients, although no functional immune responses were tested. Our data extend these findings, demonstrating improved CTL function in MR4.5 beyond that of MMR, as shown by increased LAA-positive immune responses in CML patients treated with imatinib, nilotinib, and dasatinib. However, the specific causal relationship linking achievement of DMR to enhanced immune responses or vice versa remains to be fully elucidated. In an attempt to investigate this further, we aimed to characterize the immune profile of CML patients with a “suboptimal” response to TKIs, such as failure to achieve early molecular response (BCR-ABL1 ≤10%) within the first year of therapy.50-52 However, TKIs are highly efficacious in the majority of CP-CML patients,53 supported by our data showing that 87.5% (7/8) of the pre-MMR patients in our study ultimately achieved MMR. We therefore did not have access to “suboptimal” response samples. We speculate that deeper remission on TKI potentiates MDSC and Treg reduction, possibly below a yet-undetermined threshold level, resulting in increased NK cell and T-cell effector responses and an overall improved immune response.
We found that in TKI-induced MMR and MR4.5, the most abundant LAA-specific CTL response was to PRAME, WT1, and then BMI-1, with the least immunogenic response to PR3. Burchert et al54 have previously reported imatinib-induced downregulation of PR3, which may account for the reduced PR3 responses observed in our study. The increased PR3-specific CTL response observed in healthy donors in our study (∼70%) compared with previous reports (∼40%)55 is likely from the strength of using a 15-mer peptide library screening approach, enabling determination of immunogenic CD4+ and CD8+ T-cell epitopes across the entire protein compared with other studies using a single 9-mer screening approach. We identified impaired effector CTL immune responses to all 4 LAAs in CML patients at diagnosis compared with those in MMR and MR4.5 and healthy donors; this is likely mediated via several mechanisms. CD62L downregulation may impair effector CTL immune responses in CML patients at diagnosis and abrogate antileukemic immune control because CD62L is critical in controlling the traffic of T cells to secondary lymphoid tissues and priming by APCs. Sopper et al56 have reported decreased CD62L surface expression on T cells in CML patients at diagnosis and shown that expression normalizes to that of healthy donors 6 months after nilotinib therapy. Similarly, we found CD62L expression was downregulated in CML patients at diagnosis and effectively restored to normal levels in CML patients achieving MMR and MR4.5 on imatinib, nilotinib, and dasatinib (data not shown). In addition, enhanced PD-1 signaling on activated T cells plays a critical role in acquisition of an exhaustion phenotype, which occurs as a result of persistent antigen stimulation that culminates in a loss of effector function and disease progression.57 This suggests that aberrant PD-1 signaling may also contribute to the functional T-cell impairment we observed in CML patients at diagnosis, and it is possible the restoration of PD-1 to normal levels in MR4.5, associated with concomitant recovery of LAA-CTL responses is a consequence of diminishing antigenic stimulation in light of the significantly reduced leukemic burden on TKIs. Similarly, such persistent antigen-driven stimulation may also be linked to the diminished NK cell effector function observed in CP-CML patients at diagnosis.
There are preliminary data that CML patients who maintain TFR successfully after TKI cessation have higher NK cell numbers and function compared with those who relapse off-treatment,9 further supporting the importance of recrudescence of effector-mediated immune surveillance for achieving sustained TFR in CML. Delineating the threshold value of the various immune effector responses in patients achieving DMR would help identify individual CML patients in which TKI cessation is more achievable and successful because absolute levels of BCR-ABL1 at the time of treatment cessation have not been able to predict those who are destined to relapse off-treatment.2 In conclusion, we hypothesize that in a setting of high leukemic cell load in CP-CML patients at diagnosis, immunosuppressive mechanisms predominate, mediated in part by increased MDSCs that are part of the BCR-ABL1–positive leukemic cell clone.45 MDSCs inhibit NK cell cytotoxicity, limit CTL-immune responses via the aberrantly expressed PD-1/PD-L1 pathway, and promote Treg activation and expansion.58,59 Enhanced PD-1 signaling further supports the development, maintenance, and immunosuppressive function of Tregs.60 Tregs dampen immune responses by suppressing the function of effector T and NK cells.61 When successful TKI therapy reduces the leukemic cell load, suppressor cell activity, and PD-1 expression, there is consequent reactivation of the immune effector response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the assistance of Randall Grose, Australian Cancer Research Foundation Imaging Suite, South Australia Health and Medical Research Institute, and Katherine Pilkington, Detmold Family Trust Cell Imaging Centre, SA Pathology.
This study was supported by the Royal Adelaide Hospital Contributing Haematologists’ Committee, the Sail for Cancer Research Fellowship (A.S.M.Y.), and the Australian National Health and Medical Research Council grant (APP1059165).
Authorship
Contribution: A.H. contributed to research design, collected and analyzed the data, performed statistical analysis, and wrote the manuscript; J.C. contributed to research design, performed the research, and critically reviewed the manuscript; C.T. and L.V. contributed to research design and critically reviewed the manuscript; D.L.W. and T.P.H. critically reviewed the manuscript; and A.S.M.Y. contributed to research design, supervised the research, interpreted the data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: D.L.W. and T.P.H. acted as consultants or advisors to and received research funding and honoraria from Novartis, BMS, and Ariad; A.S.M.Y. received research funding from Novartis, BMS, and Celgene and honoraria from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Amy Hughes, SAHMRI Level 5 North, Cancer Theme, PO Box 11060, Adelaide, SA 5001, Australia; e-mail: amy.hughes@sahmri.com.