Key Points
The soluble interleukin-2 receptor was identified as an only independent prognostic factor for chronic and smoldering ATL in this study.
The prognostic index using the values of sIL-2R (iATL-PI) is a promising tool for the risk-adapted therapeutic intervention.
Abstract
Adult T-cell leukemia-lymphoma (ATL) has been divided into 4 clinical subtypes: acute, lymphoma, chronic, and smoldering. The aim of this study is to develop a novel prognostic index (PI) for chronic and smoldering ATL. We conducted a nationwide retrospective survey on ATL patients, and 248 fully eligible individuals were used in this analysis. In the univariate analysis, sex, performance status, log10 (soluble interleukin-2 receptor [sIL-2R]), neutrophils count, and lymphadenopathy showed values of P < .05 in training samples. A multivariate analysis was performed on these factors, and only log10 (sIL-2R) was identified as an independent prognostic factor in training samples. Using a regression coefficient of this variable, a prognostic model was formulated to identify different levels of risk: indolent ATL-PI (iATL-PI) = 1.51 × log10 (sIL-2R [U/mL]). The values calculated by iATL-PI were divided into 3 groups using a quartile point. In the validation sample, median survival times (MSTs) were 1.6 years, 5.5 years, and not reached for patients in the high-, intermediate-, and low-risk groups, respectively (P < .0001). To make the scoring system clinically practicable, we simplified iATL-PI according to trichotomizing sIL-2R at 1000 and 6000 U/mL, using a quartile point. Patients with more than 6000 U/mL sIL-2R were categorized into the high-risk group, less than and equal to 1000 U/mL into the low-risk group, and the others into the intermediate-risk group, and MSTs were 1.6 years, not reached, and 5.5 years, respectively (P < .0001). iATL-PI has potential as a novel tool for a risk-adapted therapeutic approach.
Introduction
Human T lymphotropic virus type 1 (HTLV-I), which is transmitted through breast-feeding to infants, blood transfusions, and through sexual intercourse among adults, leads to persistent infections and causes 2 distinct types of diseases: a lymphoid malignancy known as adult T-cell leukemia-lymphoma (ATL) and a few chronic inflammatory diseases. ATL has been classified into 4 clinical subtypes: acute, lymphoma, chronic, and smoldering types, based on clinical characteristics, prognoses and the natural history of the disease.1 Patients with the chronic type have been further divided into 2 categories based on the presence or absence of either of 3 unfavorable prognostic factors: serum blood urea nitrogen (BUN) or lactate dehydrogenase (LDH) levels higher than the normal upper limit, or albumin levels lower than the normal lower limit. Patients with the acute, lymphoma, and chronic types with unfavorable prognostic factors are categorized as aggressive ATL, whereas those with the chronic type without unfavorable prognostic factors and the smoldering type are categorized as indolent ATL.2 Indolent ATL is incurable, but it generally progresses slowly. These patients are therefore recommended to be carefully monitored by watchful waiting or treated with interferon-α and zidovudine (IFN/AZT) if they are symptomatic. Chemotherapy is only applied if transformation to an aggressive type occurs.3,4 However, patients with the chronic and smoldering types present various symptoms, clinical courses, and survival rates, even with the same clinical subtype.5
Two new agents have recently exhibited efficacy in the treatment of aggressive ATL. One is a monoclonal antibody against CC chemokine receptor 4 (CCR4) mogamulizumab, which has been approved for the treatment of CCR4-positive ATL, peripheral T-cell lymphoma, and cutaneous T-cell lymphoma in Japan. A single-agent phase 2 trial in patients with relapsed aggressive ATL showed the response rate of 50%, progression-free survival of 5.3 months, and median survival time (MST) of 13.7 months.6 In the front-line treatment, a randomized phase 2 study comparing VCAP (vincristine, cyclophosphamide, doxorubicin, and prednisolone)-AMP (doxorubicin, ranimustine, and prednisolone)-VECP (vindesine, etoposide, carboplatin, and prednisolone) with or without the mogamulizumab in aggressive ATL also showed an improved response by adding mogamulizumab; the complete response rate and overall response rate were 52% and 86%, respectively.7 The other treatment is lenalidomide, which is an immunomodulatory drug with antiproliferative and antitumor potential. A phase 2 study of lenalidomide in relapsed or recurrent patients with aggressive ATL showed an overall response rate, median progression-free survival, and MST of 42%, 3.8 months, and 20.3 months, respectively.8 In terms of antiviral therapies, the effect of a combination of IFN/AZT on patients with ATL was reported in 1995 for the first time.9,10 Gill et al reported the overall response rate of 58% and MST of 3 months in 19 aggressive ATLs, including 12 patients previously treated.9 A meta-analysis reported by Bazarbachi et al showed that MSTs for the first-line treatment of IFN/AZT were 9 months, 7 months, and not reached, and overall survival (OS) at 5 years was 28%, 0%, and 100% in patients with acute, lymphoma, and chronic and smoldering ATL.11 Patients with leukemic-type ATL significantly benefited from the first-line antiviral therapy of IFN/AZT.
To provide an appropriate treatment based on a risk-adapted strategy, we previously reported the development of a prognostic index for acute- and lymphoma-type ATL.12 We here report the development of a new prognostic index for chronic- and smoldering-type ATL to establish a tool for the application of risk-adapted approaches based on clinical data from a nationwide survey.
Patients and methods
Patients
We conducted the ATL-Prognostic Index (ATL-PI) Project, a nationwide retrospective survey on patients diagnosed with ATL between January 1, 2000, and May 31, 2009, by hematologists in participating hospitals in Japan. We also collected clinical information on patients with chronic- and smoldering-type ATL newly diagnosed by dermatologists during the same period for the ATL-PI project in 3 major hospitals located in Kyushu Island, in which HTLV-1 is highly endemic. All clinical data, as well as the validity of the diagnosis of ATL, were reviewed by 2 expert hematologists. Because allogeneic hematopoietic stem cell transplantation (allo-HSCT) has a strong influence on survival, as previously reported,13-16 a separate analysis has been performed on patients, including and excluding those who had allo-HSCT.
Clinical data
We collected information on age, sex, institutional-based clinical subtype, hemoglobin, platelet counts, white blood cell counts, neutrophil counts, lymphoid cell counts, abnormal lymphoid cell counts, serum total protein, serum albumin, LDH, soluble interleukin-2 receptor (sIL-2R), BUN, C-reactive protein, maximum tumor size, B symptoms, performance status by the Eastern Cooperative Oncology Group (ECOG PS), Ann Arbor stage, the number of lesions of involved lymph nodes, and the sites and number of involved extranodal lesions at the time of diagnosis. The lymph nodes and extranodal diseases were assessed by clinical examination or imaging tests such as a computed tomography, ultrasonography, and magnetic resonance imaging. Abnormal lymphocytes showing cerebriform or flower-like nuclei were microscopically identified in each institution. We defined leukemic stage IV diseases as those presenting with more than 1% abnormal lymphocytes in peripheral blood according to the definition for diagnosing acute- and lymphoma-type ATL in Shimoyama’s classification.1 OS was calculated from the date of diagnosis to the date of death by any cause or to the last follow-up date. Time to systemic chemotherapy was calculated from the date of diagnosis to the date of introduction of systemic chemotherapy. Patients undergoing allo-HSCT were determined to be censored data, and OS was calculated from the date of diagnosis to the date of the transplantation.
Approval for the study procedure was obtained from the Ethics Committee and Institutional Review Board of the coordinating center (Fukuoka University), and each participating center supplied data according to their institutional policies. Patients gave informed consent in accordance with the Declaration of Helsinki.
Statistical analysis
Prognostic factors were identified using the method as previously described.12 First, clinical data were initially split randomly into either “training samples” for the calculation of the PI or “validation samples” for the evaluation of the PI obtained, according to institutions and clinical subtypes as stratification factors. Continuous variables were applied to the analysis without categorization in avoid the loss of information. We applied parametric models based on 2-degree fractional polynomial (FP) functions to retain relevant continuous variables. The association of each variable with OS was evaluated using a univariate FP model. The multivariate FP (MFP) model using backward elimination was applied. A continuous PI from the final MFP model was categorized into 3 risk groups (low, intermediate, and high), using a quartile point in the continuous PI. Both the data sets with and without patients undergoing allo-HSCT were subjected to these analysis. To make a clinically applicable prognostic model, a simplified PI was subsequently developed based on the variables identified by the method described here. The categorization of continuous variables led to a simplified PI.
Survival curves for the 3 risk groups were estimated using the Kaplan-Meier method, and compared with the log-rank test. All statistical analyses were performed with SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC) with %mfp8 macro13. All tests were 2-sided, and values of P < .05 were considered to be significant.
Results
We collected data from 395 patients with chronic- and smoldering-type ATL (282 from hematologists and 113 from dermatologists). Among these patients, 128 were excluded for the following reasons: 95 for loss of data, 27 for unsatisfied diagnostic criteria of ATL, 4 for double registration resulting from hospital transfer, and 2 for being diagnosed before 2000 (Figure 1). The clinical characteristics are listed in Table 1. Of 149 patients with chronic-type ATL, 106 were diagnosed as unfavorable chronic type. Among 118 patients with smoldering-type ATL, 69 had less than 5% abnormal lymphocytes in peripheral blood. Cutaneous lesions were presented in all 69 patients, and lung involvement in only 1 patient. The number of patients treated with systemic chemotherapies, which generally comprised those who exhibited transformation to aggressive ATL, was 139 (52%). The number of patients who died during the course of this study was 120 (45%), and the median observation period was 24 months. The most common causes of death were the progression of ATL (n = 86), followed by infectious complications during treatment of ATL (n = 12) and those without disease progress (n = 4). Eighteen patients died of other causes. Regarding chemotherapies, the most frequently used regimen was VCAP-AMP-VECP (36%).17,18 The second most common regimen was CHOP (combination of cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, accounting for 31%. Single-agent therapies, such as etoposide and sobuzoxane, followed at 19%, and others at 13%.
Flowchart of patients included in the analysis. The clinical information of 395 patients with chronic- and smoldering-type ATL were collected (282 from hematologists and 113 from dermatologists). Among these patients, 128 were excluded for the following reasons: 95 for loss of data, 27 for unsatisfied diagnostic criteria of ATL, 4 for double registration resulting from hospital transfer, and 2 for being diagnosed before 2000. First, the remaining patients without those undergoing allo-HSCT were randomly divided into 2 groups: training samples-A and validation samples-A. The patients, including those undergoing allo-HSCT, were subsequently divided into 2 groups: training samples-B and validation samples-B.
Flowchart of patients included in the analysis. The clinical information of 395 patients with chronic- and smoldering-type ATL were collected (282 from hematologists and 113 from dermatologists). Among these patients, 128 were excluded for the following reasons: 95 for loss of data, 27 for unsatisfied diagnostic criteria of ATL, 4 for double registration resulting from hospital transfer, and 2 for being diagnosed before 2000. First, the remaining patients without those undergoing allo-HSCT were randomly divided into 2 groups: training samples-A and validation samples-A. The patients, including those undergoing allo-HSCT, were subsequently divided into 2 groups: training samples-B and validation samples-B.
Summary of characteristics of 267 patients at diagnosis
| Characteristics . | Number/value . | % . | Range . |
|---|---|---|---|
| Age, median, years | 63.0 | 29-94 | |
| Sex | |||
| Female | 125 | 47 | |
| Male | 142 | 53 | |
| Clinical subtype | |||
| Chronic type | 149 | 56 | |
| Smoldering type | 118 | 44 | |
| Admitted to | |||
| Hematology Department | 184 | 69 | |
| Dermatology Department | 83 | 31 | |
| Neutrophil count, median, ×109/L | 4.1 | 0.0-36.6 | |
| Lymphocyte count, median, ×109/L | 4527 | 480-80 567.5 | |
| Abnormal lymphocyte count, median, ×109/L | 1554 | 0.0-78 826 | |
| Hemoglobin level, median, g/dL | 13.6 | 4.2-17.7 | |
| Platelet count, median, ×109/L | 220 | 63-560 | |
| Serum total protein, median, g/dL | 7.1 | 4.8-8.9 | |
| Serum albumin, median, g/dL | 4.2 | 2.3-5.4 | |
| BUN, median, mg/dL | 13.4 | 3.5-70.0 | |
| LDH, median, IU/L | 246 | 122-926 | |
| LDH > ULN, % | 134 | 50 | |
| Soluble IL-2R, median, U/mL | 2796 | 192-162 000 | |
| Increased CRP present | 83 | 31 | |
| Stage | |||
| I-II | 29 | 11 | |
| III-IV | 238 | 89 | |
| ECOG performance status | |||
| 0-1 | 248 | 93 | |
| 2-4 | 19 | 7 | |
| B symptoms present | 26 | 10 | |
| Number of lymph node lesions, median, range | 0 | 0-11 | |
| Number of extranodal sites, median, range | 1 | 0-4 | |
| Total number of involved lymph nodes and extranodal lesions, median, range | 4 | 0-13 | |
| Bone marrow involvement present | 39 | 15 | |
| Liver involvement present | 4 | 2 | |
| Spleen involvement present | 13 | 5 | |
| Skin lesions present | 162 | 61 | |
| Lung involvement present | 12 | 4 |
| Characteristics . | Number/value . | % . | Range . |
|---|---|---|---|
| Age, median, years | 63.0 | 29-94 | |
| Sex | |||
| Female | 125 | 47 | |
| Male | 142 | 53 | |
| Clinical subtype | |||
| Chronic type | 149 | 56 | |
| Smoldering type | 118 | 44 | |
| Admitted to | |||
| Hematology Department | 184 | 69 | |
| Dermatology Department | 83 | 31 | |
| Neutrophil count, median, ×109/L | 4.1 | 0.0-36.6 | |
| Lymphocyte count, median, ×109/L | 4527 | 480-80 567.5 | |
| Abnormal lymphocyte count, median, ×109/L | 1554 | 0.0-78 826 | |
| Hemoglobin level, median, g/dL | 13.6 | 4.2-17.7 | |
| Platelet count, median, ×109/L | 220 | 63-560 | |
| Serum total protein, median, g/dL | 7.1 | 4.8-8.9 | |
| Serum albumin, median, g/dL | 4.2 | 2.3-5.4 | |
| BUN, median, mg/dL | 13.4 | 3.5-70.0 | |
| LDH, median, IU/L | 246 | 122-926 | |
| LDH > ULN, % | 134 | 50 | |
| Soluble IL-2R, median, U/mL | 2796 | 192-162 000 | |
| Increased CRP present | 83 | 31 | |
| Stage | |||
| I-II | 29 | 11 | |
| III-IV | 238 | 89 | |
| ECOG performance status | |||
| 0-1 | 248 | 93 | |
| 2-4 | 19 | 7 | |
| B symptoms present | 26 | 10 | |
| Number of lymph node lesions, median, range | 0 | 0-11 | |
| Number of extranodal sites, median, range | 1 | 0-4 | |
| Total number of involved lymph nodes and extranodal lesions, median, range | 4 | 0-13 | |
| Bone marrow involvement present | 39 | 15 | |
| Liver involvement present | 4 | 2 | |
| Spleen involvement present | 13 | 5 | |
| Skin lesions present | 162 | 61 | |
| Lung involvement present | 12 | 4 |
BUN, blood urea nitrogen; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; IL-2R, interleukin-2 receptor; ULN, upper limit of normal.
The sIL-2R level by picogram per milliliter may be converted to units per milliliter, using the formula: value (pg/mL) × 0.113.
Development of indolent ATL-PI
First, the patients, excluding those who had allo-HSCT, were randomly divided into 2 groups: training and validation samples-A. The patients, including those undergoing allo-HSCT, were subsequently divided into 2 groups: training and validation samples-B. A univariate analysis was performed on training samples-A, and the following variables showed values of P < .05: sex, ECOG PS, neutrophil count, log10 (sIL-2R) (shown in log scale because it followed a nonlinear function), and number of lymph node lesions (Table 2). We then applied the MFP model, using these 5 factors and Shimoyama’s classification because it is a well-known prognostic criteria, and only log10 (sIL-2R) was identified as an independent factor (hazard ratio, 4.5443; P < .0001). The linear risk function based on Cox’s regression coefficients was as follows: indolent ATL-PI (iATL-PI) = 1.51 × log10 (sIL2R [U/mL]). The median of iATL-PI in training samples-A was 5.17 (range, 3.45-7.28). The 25 percentile line of the values corresponded to 4.62, and the 75 percentile line to 5.79. These values were used as cutoff points to classify patients into the 3 risk groups: patients with a score ≤4.62 belonged to the low-risk group, 4.62 < score ≤ 5.79 to the intermediate-risk group, and score >5.79 to the high-risk group. iATL-PI was applied to validation samples-A to clarify its accuracy and reproducibility. The numbers of patients classified into the low-, intermediate-, and high-risk groups were 34, 66, and 24, respectively. MSTs were not reached (95% confidence interval [CI], 6.2-), 5.5 (95% CI, 2.5-), and 1.6 years (95% CI, 0.7-3.5), and OS rates at 4 years were 77.2% (95% CI, 59.2%-88.7%), 54.3% (95% CI, 38.7%-69.1%), and 17.3% (95% CI, 4.4%-48.2%) in patients in the low-, intermediate-, and high-risk groups, respectively (P < .0001, log-rank test; Figure 2A).
Results of variable selection by the MFP model in training samples-A
| Variates . | Univariate analysis . | Multivariate analysis . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | Regression coefficient . | |||
| Lower . | Upper . | Lower . | Upper . | ||||||
| Sex | |||||||||
| Male | 1.00 | ||||||||
| Female | 0.5092 | 0.2902 | 0.8933 | .0186 | |||||
| ECOG performance status | |||||||||
| 0-1 | 1.00 | ||||||||
| 2-4 | 3.0622 | 1.4447 | 6.4904 | .0035 | |||||
| Neutrophil count | |||||||||
| Continuous | 1.0001 | 1.0000 | 1.0001 | .0020 | |||||
| log10(sIL2R [U/mL]) | |||||||||
| Continuous | 4.5443 | 2.7466 | 7.5187 | <.0001 | 4.5443 | 2.7466 | 7.5187 | <.0001 | 1.5139 |
| Number of lymph node lesions | |||||||||
| Continuous | 1.1936 | 1.0462 | 1.3617 | .0085 | |||||
| Shimoyama’s classification | |||||||||
| Smoldering | 1.0000 | ||||||||
| Chronic | 1.6130 | 0.9626 | 2.7027 | .0695 | |||||
| Variates . | Univariate analysis . | Multivariate analysis . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | Regression coefficient . | |||
| Lower . | Upper . | Lower . | Upper . | ||||||
| Sex | |||||||||
| Male | 1.00 | ||||||||
| Female | 0.5092 | 0.2902 | 0.8933 | .0186 | |||||
| ECOG performance status | |||||||||
| 0-1 | 1.00 | ||||||||
| 2-4 | 3.0622 | 1.4447 | 6.4904 | .0035 | |||||
| Neutrophil count | |||||||||
| Continuous | 1.0001 | 1.0000 | 1.0001 | .0020 | |||||
| log10(sIL2R [U/mL]) | |||||||||
| Continuous | 4.5443 | 2.7466 | 7.5187 | <.0001 | 4.5443 | 2.7466 | 7.5187 | <.0001 | 1.5139 |
| Number of lymph node lesions | |||||||||
| Continuous | 1.1936 | 1.0462 | 1.3617 | .0085 | |||||
| Shimoyama’s classification | |||||||||
| Smoldering | 1.0000 | ||||||||
| Chronic | 1.6130 | 0.9626 | 2.7027 | .0695 | |||||
95% CI, 95% confidence interval; HR, hazard ratio.
OS curves for validation samples. (A) OS curves for validation samples-A according to iATL-PI. iATL-PI was calculated as 1.51 × log10 (sIL2R [U/mL]). Patients were stratified according to the following 3 risk groups: low risk, score ≤4.62; intermediate risk, 4.62 < score ≤ 5.79; and high risk, score >5.79. (B) OS curves for validation samples-A according to simplified iATL-PI. Patients were stratified according to the following 3 risk groups: low risk, sIL-2R ≤1000; intermediate risk, 1000 < sIL-2R ≤ 6000; and high risk, sIL-2R > 6000. (C) OS curves for validation samples-B according to Shimoyama’s classification, including the favorable/unfavorable chronic types and smoldering type. The sIL-2R level by picogram per milliliter may be converted to units per milliliter, using the formula: value (pg/mL) × 0.113.
OS curves for validation samples. (A) OS curves for validation samples-A according to iATL-PI. iATL-PI was calculated as 1.51 × log10 (sIL2R [U/mL]). Patients were stratified according to the following 3 risk groups: low risk, score ≤4.62; intermediate risk, 4.62 < score ≤ 5.79; and high risk, score >5.79. (B) OS curves for validation samples-A according to simplified iATL-PI. Patients were stratified according to the following 3 risk groups: low risk, sIL-2R ≤1000; intermediate risk, 1000 < sIL-2R ≤ 6000; and high risk, sIL-2R > 6000. (C) OS curves for validation samples-B according to Shimoyama’s classification, including the favorable/unfavorable chronic types and smoldering type. The sIL-2R level by picogram per milliliter may be converted to units per milliliter, using the formula: value (pg/mL) × 0.113.
The univariate and multivariate analysis on the data sets including patients treated with allo-HSCT (sample-B) were subsequently performed, and sex, ECOG PS, neutrophil count, log10 (sIL-2R), and number of lymph node lesions showed P values lower than .05. We then applied the multivariate MFP model using these 5 factors and Shimoyama’s classification. Finally, only log10 (sIL-2R) remained significant, just like the analysis without patients undergoing allo-HSCT (supplemental Table 1, available on the Blood Web site). The linear risk function based on Cox’s regression coefficients was as follows: 1.53 × log10 (sIL2R [U/mL]). The median of this prognostic index in training samples-B was 5.27 (range, 3.49-7.97). The 25 percentile line of the values corresponded to 4.68, and the 75 percentile line to 5.83. These values were used as cutoff points to classify patients into the 3 risk groups: patients with a score ≤4.68 belonged to the low-risk group, 4.68 < score ≤ 5.83 to the intermediate-risk group, and score >5.83 to the high-risk group. This prognostic index was applied to validation samples-B. The numbers of patients classified into the low-, intermediate-, and high-risk groups were 34, 69, and 29, respectively. MSTs were not reached (95% CI, 6.2-), 5.5 (95% CI, 3.2-), and 1.6 years (95% CI, 0.6-3.5), and OS rates at 4 years were 76.4% (95% CI, 58.1%-88.3%), 57.2% (95% CI, 41.4%-71.5%), and 20.0% (95% CI, 5.8%-50.4%) in patients in the low-, intermediate-, and high-risk groups, respectively (P < .0001, log-rank test) (supplemental Figure 1). These results indicated that sIL-2R was a good prognostic marker in patients with chronic- and smoldering-ATL, including those treated with allo-HSCT.
Development of a simplified iATL-PI
To make a clinically applicable prognostic model, we established a simplified iATL-PI, which was developed on the basis of the original iATL-PI with some modifications. The values of sIL-2R, which were identified as a continuous prognostic factor by the MFP model, were classified into 3 risk groups using a quartile point. The 25 percentile line of the values corresponded to 1156 U/mL, and the 75 percentile line to 6835 U/mL. We employed the values of 1000 and 6000 U/mL as cutoff points to classify patients into the 3 risk groups: low risk; sIL-2R ≤1000 U/mL; intermediate risk, 1000 U/mL < sIL-2R ≤ 6000 U/mL; and high risk; sIL-2R, >6000 U/mL. The simplified iATL-PI was then applied to validation samples-A, which showed that the numbers of patients in the low-, intermediate-, and high-risk groups were 30, 64, and 30, respectively. MSTs were not reached (95% CI, 4.6-), 5.5 (95% CI, 3.3-), and 1.6 years (95% CI, 0.7-3.5), and OS rates at 4 years were 77.6% (95% CI, 58.3%-89.6%), 55.6% (95% CI, 40.0%-70.4%), and 26.2% (95% CI, 10.3%-51.0%) in patients in the low-, intermediate-, and high-risk groups, respectively, in the validation samples-A (Figure 2B). The simplified iATL-PI was also applied to the validation samples-B, including patients undergoing allo-HSCT (n = 132). MSTs were not reached (95% CI, 4.6-), 5.5 (95% CI, 3.1-), and 1.4 years (95% CI, 0.7-2.6), and OS rates at 4 years were 77.6% (95% CI, 58.3%-89.6%), 54.1% (95% CI, 39.3%-68.2%), and 22.1% (95% CI, 8.8%-45.6%) in patients in the low-, intermediate-, and high-risk groups, respectively (P < .0001, log-rank test). The simplified iATL-PI allowed us to classify patients with chronic- and smoldering-type ATL accurately into the 3 different prognostic groups, as well as the original iATL-PI.
In addition, the simplified iATL-PI was applied to the data set (n = 83) of patients being treated by dermatologists. MSTs were not reached (95% CI, 3.9-), 5.5 (95% CI, 2.3-), and 1.8 years (95% CI, 0.4-), and OS rates at 4 years were 68.1% (95% CI, 47.7%-83.3%), 60.1% (95% CI, 42.1%-74.5%), and 35.6% (95% CI, 10.1%-73.1%) in patients in the low-, intermediate-, and high-risk groups, respectively (supplemental Figure 2).
Comparison with previously reported prognostic factors
Several prognostic factors have previously been reported in patients with chronic- or smoldering-type ATL (ie, age, sex, B symptoms, PS, neutrophil count, serum albumin, LDH, BUN, and total number of involved lymph nodes and extranodal lesions).2,19,20 The median values of these previous factors were calculated from the risk group classified by the simplified iATL-PI (supplemental Table 2). Most factors, except for age and sex, correlated with the current classification.
We compared the discrimination of prognoses between the simplified iATL-PI and Shimoyama’s classification; namely, favorable chronic, unfavorable chronic, and smoldering types, with the validation samples-B. The power to discriminate prognoses was markedly better by the simplified iATL-PI than Shimoyama’s classification (Figure 2C). The distribution of patients classified by the simplified iATL-PI was also compared with that by Shimoyama’s classification (Table 3). It is important to note that 7.6% of patients with smoldering type and 11.6% of patients with the favorable chronic type, which are recommended by the current standard of care in Japan to be monitored by watchful waiting without the introduction of chemotherapy, were recognized as high risk. Importantly, all but 1 of these patients were initiated systemic chemotherapy within 2 years of their diagnoses. In contrast, 39.6% of patients with the unfavorable chronic type were distributed in the intermediate-risk group.
Comparison between Shimoyama’s classification and the simplified iATL-PI
| . | Shimoyama’s classification . | |||||
|---|---|---|---|---|---|---|
| Smoldering type . | Favorable chronic type . | Unfavorable chronic type . | ||||
| n . | % . | n . | % . | n . | % . | |
| iATL-PI | ||||||
| Low risk | 42 | 35.6 | 8 | 18.6 | 1 | 1.0 |
| Intermediate risk | 67 | 56.8 | 30 | 69.8 | 42 | 39.6 |
| High risk | 9 | 7.6 | 5 | 11.6 | 63 | 59.4 |
| Total | 118 | 100.0 | 43 | 100.0 | 106 | 100.0 |
| . | Shimoyama’s classification . | |||||
|---|---|---|---|---|---|---|
| Smoldering type . | Favorable chronic type . | Unfavorable chronic type . | ||||
| n . | % . | n . | % . | n . | % . | |
| iATL-PI | ||||||
| Low risk | 42 | 35.6 | 8 | 18.6 | 1 | 1.0 |
| Intermediate risk | 67 | 56.8 | 30 | 69.8 | 42 | 39.6 |
| High risk | 9 | 7.6 | 5 | 11.6 | 63 | 59.4 |
| Total | 118 | 100.0 | 43 | 100.0 | 106 | 100.0 |
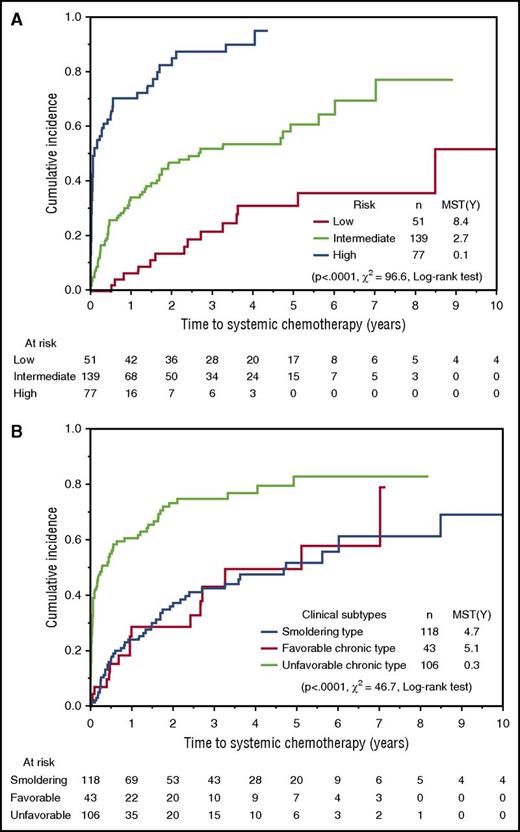
We compared periods from the diagnosis until the initiation of systemic chemotherapy between risk groups based on iATL-PI and Shimoyama’s classification. Risk groups according to iATL-PI were clearly stratified into 3 layers and showed that the median times to systemic chemotherapy in the low-, intermediate-, and high-risk groups were 8.4, 2.7, and 0.1 years, respectively (Figure 3A). In contrast, no difference in the median times to systemic chemotherapy was observed between patients with smoldering type and favorable chronic type by Shimoyama’s classification (Figure 3B). Among patients with the unfavorable chronic type, the period from the diagnosis until the initiation of systemic chemotherapy was significantly longer in the intermediate-risk group than in the high-risk group (the median times to treatment were 1.8 and 0.06 years, respectively; supplemental Figure 3).These results imply that iATL-PI has potential as a predictive model for disease progression, as well as a prognostic model.
Cumulative incidence of the introduction of systemic chemotherapy for training and validation samples-B. (A) Time from the diagnosis to introduction of systemic chemotherapy, according to the simplified iATL-PI. (B) Time from the diagnosis to introduction of systemic chemotherapy according to Shimoyama’s classification (ie, favorable/unfavorable chronic types and smoldering type).
Cumulative incidence of the introduction of systemic chemotherapy for training and validation samples-B. (A) Time from the diagnosis to introduction of systemic chemotherapy, according to the simplified iATL-PI. (B) Time from the diagnosis to introduction of systemic chemotherapy according to Shimoyama’s classification (ie, favorable/unfavorable chronic types and smoldering type).
Discussion
Several clinical prognostic models for lymphoid neoplasms have been proposed to date.21-26 Regarding patients with ATL, we previously reported ATL-PI for acute- and lymphoma-type ATL, whereas no prognostic model has been available for the chronic and smoldering types until now. In the present study, we established a novel prognostic index for these 2 clinical subtypes of ATL.
An international consensus meeting recommended multiagent chemotherapies or IFN/AZT, with or without subsequent allo-HSCT, for patients with aggressive ATL (ie, acute, lymphoma, and unfavorable chronic types) as first-line treatment.3 We recently revealed that most patients with acute- and lymphoma-type ATL received systemic chemotherapy soon after their diagnoses; however, approximately 40% of patients with the unfavorable chronic type were not subjected to systemic chemotherapy for more than 1 year from diagnoses. In indolent ATL (ie, the smoldering and favorable chronic types), the period from diagnosis to the administration of systemic chemotherapy was longer than that of the unfavorable chronic type, and markedly varied in length.5 These results suggest that the heterogeneity of the clinical course influences the treatment decision by physicians. Therefore, a novel alternative prognostic model to be used for the therapeutic stratification of these patients is required for standardized treatment.
Based on the multivariate analysis, the level of serum sIL-2R was identified as the only independent prognostic factor. Serum sIL-2R levels are measured by ELISA assay. It might not be commonly used in North America and Europe, although it has been reported that the elevated serum levels of sIL-2R have correlated with poor prognoses in a number of different types of lymphoid malignancies, particularly B-cell lymphoma, including diffuse large B-cell lymphoma and follicular lymphoma.27-30 In Japan, it is included in the current routine practice of examinations for patients with lymphoma. The soluble form of IL-2R is naturally released by proteolytic shedding from activated T cells. The sIL-2R and IL-2 complex has been reported to promote T-cell differentiation toward regulatory T-cells in follicular lymphoma; therefore, sIL-2R may contribute to a poor prognosis.30 In contrast, ATL cells are characterized by the abundant expression of the α-chain of IL-2 (CD25) on their surface. The level of serum sIL-2R is useful for assessing the tumor burden, with significantly high levels in patients with ATL and disease deterioration.12,31-35
iATL-PI enabled the definite discrimination of patients into 3 risk groups of different survival times. Furthermore, the ability of iATL-PI was superior to that of Shimoyama’s classification in our data set (Figure 2). Therefore, iATL-PI may result in a simpler and more appropriate risk-stratified approach for the treatment of chronic- and smoldering-type ATL. We propose that patients in the high-risk group be treated with conventional chemotherapy or IFN/AZT, similar to that for aggressive ATL; those in the intermediate-risk group be subjected to investigational treatments such as IFN/AZT or included in clinical trials; and those in the low-risk group be monitored by watchful waiting. A prospective study by the Japan Clinical Oncology Group to compare IFN/AZT with watchful waiting for indolent ATL is currently ongoing. Regarding the novel agents, the findings of a phase 2 study on lenalidomide showed a promising overall response rate and survival in relapsed and recurrent patients with aggressive ATL.8 Furthermore, several clinical trials on novel agents such as the histone deacetylase inhibitors, alemtuzumab, brentuximab vedotin, nivolumab, and an EZH1/2 dual inhibitor are currently in progress. This novel prognostic model would be useful for risk-adapted therapeutic intervention in the future.
We developed a new prognostic index for chronic- and smoldering-type ATL, iATL-PI, based on clinical data from a nationwide survey, which is the largest ever data set obtained on ATL. The level of sIL-2R was identified as the only independent prognostic factor using a multivariate analysis for chronic- and smoldering-type ATL. The limitation of this study is possible interference by therapeutic interventions on the survival of patients because the patients included in this study received diverse medical procedures varying from watchful waiting to aggressive chemotherapy. Therefore, this prognostic model needs to be validated prospectively in an independent population of patients. It is imperative that future studies on patients with ATL incorporate correlative studies on clinical and biological markers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yukimi Ito, Noriko Gushima, Kazuko Nakata, Yasuko Koga, Emi Ageishi, Etsuko Kumakawa, and Noriko Ikoma for supporting this study through data management and manuscript preparation.
This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI) grants 16K19580 and 16KK0206 (H.K.), by Health and Labor Sciences Research Grants for Clinical Research (H23-rinkensui-ippan-011, H26-kakushintekigann-ippan-136), for Cancer Research (H23-gan rinsho-ippan-020 and 022, H25-gan rinsho-ippan-011) from the Ministry of Health, Labour and Welfare of Japan (K.I.), and by the Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and Development, AMED (K.I.).
The authors thank the following investigators for their participation in the study: Y. Uchida (Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan), Y. Saburi (Oita Prefectural Hospital, Oita, Japan), N. Uike (National Kyushu Cancer Center, Fukuoka, Japan), Y. Ohno (Kitakyushu Municipal Medical Center, Kitakyushu, Japan), H. Matsuoka (Koga General Hospital, Miyazaki, Japan), S. Okamura (National Hospital Organization Kyushu Medical Center, Fukuoka, Japan), M. Ogata (Oita University Hospital, Yufu, Japan), T. Murayama (Hyogo Cancer Center, Akashi, Japan), M. Nakagawa (Nissay Hospital, Osaka, Japan), S. Iida (Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan), H. Nagoshi (Yokohama City Seibu Hospital, St. Marianna University School of Medicine, Yokohama, Japan), H. Teshima (Osaka City General Hospital, Osaka, Japan), H. Take (Toyonaka Municipal Hospital, Toyonaka, Japan), K. Nosaka (Kumamoto University Hospital, Kumamoto, Japan), H. Tsuda (Kumamoto Municipal Hospital, Kumamoto, Japan), N. Harada (Kyushu University Graduate School of Medical Science, Fukuoka, Japan), N. Uoshima (Matsushita Memorial Hospital, Moriguchi, Japan), J. Tsukada (University of Occupational and Environmental Health, Kitakyushu, Japan), Y. Yakushijin (Ehime University Graduate School of Medicine, Toon, Japan), H. Mori (Showa University Fujigaoka Hospital, Yokohama, Japan), Y. Abe (Kyushu University Graduate School of Medical Science, Fukuoka, Japan), T. Okamura (Kurume University School of Medicine, Kurume, Japan), Y. Kubuki (Miyazaki University, Miyazaki, Japan), Y. Yamano (Kyushu Kosei-nenkin Hospital, Kitakyushu, Japan), T. Kakimoto (Yokohama Municipal Citizen's Hospital, Yokohama, Japan), T. Kamimura (Harasanshin General Hospital, Fukuoka, Japan), M. Koike (Juntendo University Shizuoka Hospital, Izunokuni, Japan), Y. Adachi (Kobe Central Hospital of Insurance, Kobe, Japan), K. Hodohara (Shiga University of Medical Science Hospital, Otsu, Japan), M. Yamaguchi (Minoh City Hospital, Minoh, Japan), T. Murase (Nishio Municipal Hospital, Nishio, Japan), K. Ohbayashi (Toyota Memorial Hospital, Toyota, Japan), F. Kawano (National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan), A. Miyata (Chugoku Chuo Hospital, Fukuyama, Japan), T. Miyake (Asahikawa City Hospital, Asahikawa, Japan), S. Tamaki (Yamada Red Cross Hospital, Ise, Japan), R. Tabata (Hyogo Prefectural Tsukaguchi Hospital, Amagasaki, Japan), M. Iwahashi (Saiseikai-Hita Hospital, Hita, Japan), K. Izutsu (NTT Kanto Medical Center, Tokyo, Japan), M. Shinohara (Anan Kyoei Hospital, Anan, Japan), S. Motomura (Kyushu University Beppu Hospital, Beppu, Japan), S. Okamoto (Keio University School of Medicine, Tokyo, Japan), Y. Maeda (Kinki University School of Medicine, Higashiosaka, Japan), S. Ito (Iwate Medical University School of Medicine, Morioka, Japan), K. Kitamura (Ichinomiya Municipal Hospital, Ichinomiya, Japan), T. Okamoto (Takarazuka City Hospital, Takarazuka, Japan), M. Morioka (Aiiku Hospital, Sapporo, Japan), T. Shimomura (National Hospital Organization Hiroshima-Nishi Medical Center, Otake, Japan), T. Hayashi (Tenri Hospital, Tenri, Japan), H. Matsubara (Shinkokura Hospital, Kitakyushu, Japan), C. Hashimoto (Kanagawa Cancer Center, Yokohama, Japan), T. Takeichi (Health Insurance Naruto Hospital, Naruto, Japan), A. Horikoshi (Nihon University School of Medicine, Nerima-Hikarigaoka Hospital, Tokyo, Japan), A. Wakita (East Medical Center Higashi Municipal Hospital, Nagoya, Japan), F. Urase (Sakai Hospital Kinki University Faculty of Medicine, Sakai, Japan), N. Hirase (Kyushu Rosai Hospital, Kitakyushu, Japan), Y. Masaki (Kanazawa Medical University, Ishikawa, Japan), N. Sakai (Iwate Prefectural Iwai Hospital, Ichinoseki, Japan), Y. Yamanaka (Akita University Graduate School of Medicine, Akita, Japan), T. Sakura (Saiseikai Maebashi Hospital, Maebashi, Japan), M. Tsukaguchi (Sakai Municipal Hospital, Sakai, Japan), J. Tanabe (Fujieda Municipal General Hospital, Fujieda, Japan), T. Takahashi (Tenshi Hospital, Sapporo, Japan), A. Itoh (JA Shizuoka Kohseiren Enshu Hospital, Hamamatsu, Japan), H. Kaneko (Aiseikai Yamashina Hospital, Kyoto, Japan), M. Iino (Yamanashi Prefectural Central Hospital, Kofu, Japan), H. Kimura (Northern Fukushima Medical Center, Fukushima, Japan), S. Matsuda (Ohta Nishinouchi Hospital, Koriyama, Japan), H. Ifuku (Amagasaki Chuou Hospital, Amagasaki, Japan), and K. Sato (Asahikawa Medical University, Asahikawa, Japan).
Authorship
Contribution: H.K. and K.I. designed and performed the study and collected and analyzed the data; M.A., A.U., R.H., S.H., T.J., K. Tsukasaki, Y.M., E.S., S.Y., H.S., M.M., K.Y., and T.E. collected the data; M.S. analyzed the data; K.K. designed the study and collected the data; J.S. and K. Tamura designed and performed the study; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: K.I. has received honoraria from Chugai Pharma, Bristol-Myers Squibb, Celgene, Kyowa Hakko Kirin, Takeda, and Pfizer; consulted for Celgene; and received research funding from Takeda, Chugai Pharma, MSD, Kyowa Hakko Kirin, Taiho Pharma, Eisai, Novartis, Yakult, and Japan Blood Products Organization. K.K. has consulted for Minophagen Pharma. A.U. has received honoraria from Japan Blood Products Organization, Roche Diagnostics, Daiichi Sankyo, Siemens, Bristol-Myers Squibb, Pfizer, Astellas Pharma, Kyowa Hakko Kirin, Novartis Pharma, HUYA Bioscience International, Nippon Shinyaku, Chugai Pharma, and Celgene and received research funding from Kyowa Hakko Kirin (to the institution). T.J. has received honoraria from Celgene, Nippon Shinyaku, and Bristol-Myers Squibb and research funding from Handai Biken (to the institution). K. Tsukasaki has received honoraria from Zenyaku Kogyo, HUYA Bioscience, Celgene, and Chugai Pharma; consulted for Ono Pharmaceutical; received research funding from Chugai Pharma, Takeda, Celgene, and Mundipharma; and received travel expenses from Mundipharma. Y.M. has received research funding from Celgene (to the institution). S.Y. has received research funding from Kyowa Hakko Kirin (to the institution). J.S. has received speakers’ bureau funding from Chugai Pharma, Eisai, Bristol-Myers Squibb, and Takeda, and research funding from Kyowa Hakko Kirin, Chugai Pharma, Astellas Pharma, Toyama Chemical, Taiho Pharma, Yakult, Pfizer, and Eisai. K. Tamura has received honoraria from Ono Pharma, Asahikasei Pharma, and Lilly; consulted for Kyowa Hakko Kirin and Celgene; and received research funding from Sumitomo Dainippon Pharma, Nippon Kayaku, Shionogi, and Eisai. The remaining authors declare no competing financial interests.
Correspondence: Kenji Ishitsuka, Division of Hematology and Immunology, Center for Chronic Viral Diseases, Graduate School of Medical and Dental Sciences, Kagoshima University, 8-35-1 Sakuragaoka, Kagoshima 890-8544, Japan; e-mail: kenji-i@m.kufm.kagoshima-u.ac.jp.

![Figure 2. OS curves for validation samples. (A) OS curves for validation samples-A according to iATL-PI. iATL-PI was calculated as 1.51 × log10 (sIL2R [U/mL]). Patients were stratified according to the following 3 risk groups: low risk, score ≤4.62; intermediate risk, 4.62 < score ≤ 5.79; and high risk, score >5.79. (B) OS curves for validation samples-A according to simplified iATL-PI. Patients were stratified according to the following 3 risk groups: low risk, sIL-2R ≤1000; intermediate risk, 1000 < sIL-2R ≤ 6000; and high risk, sIL-2R > 6000. (C) OS curves for validation samples-B according to Shimoyama’s classification, including the favorable/unfavorable chronic types and smoldering type. The sIL-2R level by picogram per milliliter may be converted to units per milliliter, using the formula: value (pg/mL) × 0.113.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/1/10.1182_blood-2017-01-757542/4/m_blood757542f2.jpeg?Expires=1769094208&Signature=0rUWLiSaD2~NOq3sQTA2bR4T1drAIAwySXBHDEYkOH2e2FH34YxSjNjzC7bwxc~MWYkNFuhq2wClnxIdqqbiYOm4PadYKDORiGvs2BRMEivPmE9c8uro4iov5w323Yx3xIMokgblUHWQOW15upISp9UmIZ~5uZyHQsq-S~CJD~KGfVxBg-STNgNrgKg-rUoOkIB16752rXAcSdNykXm2c4-37q7P056WRjuqcWUCn05EpLF4so0ZGjMP4VTgLq4gsnmOvpUtfxavqbJ-gIVyHpuPitlnwP1gXRhr9VFsmx33mKX5~OcQUpkROjv9A6zfBFBaHmGBWY67u~xOJpqCcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal