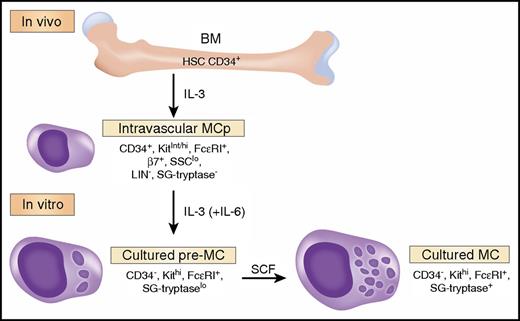
The study of cytokine requirements for development of a granulated human mast cell (MC) from circulating bone marrow (BM)-derived progenitors (MCp) by Dahlin et al,1 in this issue of Blood, identifies an interleukin-3 (IL-3)-dependent viability and maturation stage distinct from the subsequent stem cell factor (SCF)-induced secretory granule (SG) mature stage (see figure). IL-3 alone was known to be insufficient for development of identifiable human MC, and the consensus opinion for their in vitro generation used the cytokine combination of SCF, IL-6, and IL-3.2 Dahlin et al noted that MCp, identified as CD45int, CD34+, SSClo, Lin−, CD117int/hi (Kit), and FceR1+ cells, were present in low numbers in normal subjects. Others had observed that patients treated with imatinib, which blocks membrane protein kinases including the KIT receptor, became MC deficient.3 When Dahlin et al found that MCp were not reduced in patients treated with imatinib, they concluded that SCF was not needed for MCp development. They next cultured MCp with IL-3 + IL-6, noting prolonged survival with early signs of maturation, and they termed these cultured cells pre-MC. The IL-3 + IL-6-induced development of pre-MC from MCp was associated with downregulation of CD34 and β7 integrin and appearance of SG metachromasia and tryptase immunodetection. This conversion occurred even in the presence of imatinib or anti-SCF, establishing that Kit signaling played no role in this early maturation of MCp to pre-MC. Whereas IL-3 alone promoted survival of IL-3 + IL-6-generated pre-MC, IL-6 had no effect and was not further assessed. Thus, the authors used in vivo data to highlight the Kit independence of MCp and then characterized a distinct MCp-derived intermediate stage of maturation, pre-MC, on the path to full SCF-mediated maturation in culture.
MC are derived from hematopoietic stem cells in the BM. A committed, small, agranular progenitor (MCp) has been identified in the human vasculature that requires IL-3 for its survival and initial differentiation. In the presence of IL-3 (and IL-6), isolated MCp can be driven to further differentiate into an intermediate form termed a pre-MC. These latter cells can be further driven to a more mature state by culture with SCF, characterized by more mature SG and expressing a surface phenotype similar to MC isolated from human tissue. We have limited the phenotypic markers to the data presented in the article by Dahlin et al but assume that maturation changes for SG would be progressive, whereas β7 integrin (β7) expression declines. Professional illustration by Somersault18:24.
MC are derived from hematopoietic stem cells in the BM. A committed, small, agranular progenitor (MCp) has been identified in the human vasculature that requires IL-3 for its survival and initial differentiation. In the presence of IL-3 (and IL-6), isolated MCp can be driven to further differentiate into an intermediate form termed a pre-MC. These latter cells can be further driven to a more mature state by culture with SCF, characterized by more mature SG and expressing a surface phenotype similar to MC isolated from human tissue. We have limited the phenotypic markers to the data presented in the article by Dahlin et al but assume that maturation changes for SG would be progressive, whereas β7 integrin (β7) expression declines. Professional illustration by Somersault18:24.
A multiple step pathway for the development of BM-derived human MC mimics the in vitro and in vivo studies of MC development in the mouse, which have the advantage of analyses using mutant strains. Initial studies of MCp in BM and peripheral tissues used simple limiting dilution analysis of isolated mononuclear cells and cytokine clonal expansion with maturation to obtain the numbers of BM-derived MCp per million mononuclear cells in the tissue.4,5 Mouse BM-derived MC, termed BMMC, developed with IL-3 alone became a standard cell for biochemical studies, including the subsequent demonstration of the profound maturation and SG granulation effect of SCF.6 Most importantly, BMMC developed normally with IL-3 using BM from strains lacking KIT or SCF, clearly indicating a separate MC initial development stage not dependent on SCF,7 as now shown by Dahlin et al in the human.
A second similarity between human and mouse is the downregulation of β7 integrin with maturation. In the mouse, β7 integrin expression by MCp is essential for their normal transendothelial migration to jejunum or for their recruitment to lung with Th2 inflammation.5 Subsequent analysis of in vivo MC development with Th2 inflammation in lung using flow cytometry showed that recruitment of MCp was followed by early and ongoing maturation with progressive increases in SG granulation and reductions in β7 integrin and Kit expression.8 In addition, Chen et al9 used β7 integrin expression to identify an MCp-derived early maturation state generating IL-9 that is key to the MC-dependent model studied. That conventional histologic techniques are directed to mature SG means developing stages are overlooked along with possible functions.
The similarity of development pathways for mouse and human for BMMC is reassuring especially because Th2 is a driver for induced adaptive MC expansion. Both humans and mice also have innate MC that are developed from yolk sac/liver progenitors during gestation in a T-cell–independent manner. Although the adaptive reside in mucosa, the innate occupy submucosal sites in most tissues. The innate are ancient in evolution and precede the appearance of adaptive immunity. A recent transcriptomic analysis of the connective tissue mouse MC in skin and other sites showed a distinct signature with significant overlap for human skin MC, whereas there was no significant overlap with basophils.10 That both MC subclasses of mouse and human line up rather well suggests that much has yet to be learned about their functions and the purpose of 2 subclasses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal