Key Points
SCF and KIT signaling are dispensable for the survival, proliferation, and maturation of human mast cell progenitors.
Abstract
Human hematopoietic progenitors are generally assumed to require stem cell factor (SCF) and KIT signaling during differentiation for the formation of mast cells. Imatinib treatment, which inhibits KIT signaling, depletes mast cells in vivo. Furthermore, the absence of SCF or imatinib treatment prevents progenitors from developing into mast cells in vitro. However, these observations do not mean that mast cell progenitors require SCF and KIT signaling throughout differentiation. Here, we demonstrate that circulating mast cell progenitors are present in patients undergoing imatinib treatment. In addition, we show that mast cell progenitors from peripheral blood survive, mature, and proliferate without SCF and KIT signaling in vitro. Contrary to the prevailing consensus, our results show that SCF and KIT signaling are dispensable for early mast cell development.
Introduction
Mast cells are well known for their roles in immunoglobulin E (IgE)–mediated allergic disorders. In such disorders, allergens and allergen-specific IgE cross-link the high-affinity IgE receptors on the mast cell surface. The cross-linking causes the mast cells to release bioactive compounds into the microenvironment, thus resulting in an inflammatory reaction. Mast cells are also involved in the pathogenesis of systemic mastocytosis, a disease characterized by the infiltration of atypical mast cells in different tissues.
The growth factor required to generate human mast cells was unknown until the early 1990s. Attempts to derive human mast cells using interleukin-3 (IL-3) have been unsuccessful,1-3 even though IL-3 promotes mouse mast cell growth and differentiation in vitro.4 The discovery and cloning of stem cell factor (SCF), a growth factor that strongly stimulates human mast cell generation in vitro, has revolutionized the mast cell field.5-12 As a result, it is generally believed that SCF is required during the differentiation of human mast cells.13,14
Human CD34+ bone marrow progenitor cells give rise to all blood cell types, including mast cells. The mast cell progenitors from the bone marrow enter the blood circulation, and there are identified as cells expressing CD34, the SCF receptor KIT (CD117), and the IgE receptor FcεRI and lacking the expression of lineage markers.15 Full maturation of mast cell progenitors takes place in the peripheral organs; consequently, mature mast cells are virtually undetectable in the blood under normal conditions.16-19 In vitro, mast cells can be generated from progenitor cells of various origins, including bone marrow, peripheral blood, fetal liver, and cord blood.2,3,20,21 SCF is sufficient for mast cell generation in in vitro cultures in all the previously mentioned cases. However, IL-6 is frequently included in the culture medium throughout the culture period to enhance SCF-dependent mast cell proliferation and maturation.22,23 Some protocols use IL-6 and SCF-containing medium supplemented with IL-3 in the beginning of the culture. Nonetheless, whether supplementation of IL-3 early in the culture has an effect on the growth and maturation of mast cells is controversial.24
The importance of SCF and KIT signaling in the generation of mast cells has been investigated in both murine and human model systems. Wsh/Wsh and W/Wv mice, which have profound defects in KIT signaling, lack mast cells.25,26 Similarly, Sl/Sl-d mice, which lack the membrane-bound form of SCF, are mast cell deficient.27 However, mast cells can be generated in vitro from wild-type mice, in mice with defective KIT signaling, and in mice lacking membrane-bound SCF using IL-3.28 Furthermore, the perfusion of IL-3 almost completely restores the cutaneous mast cell compartment in W/Wv mice.29 SCF and KIT signaling are therefore dispensable for the differentiation of mast cells in mice in vitro and in vivo, and IL-3 can substitute for the role of SCF. In humans, disruption of KIT signaling through treatment with the tyrosine kinase inhibitor imatinib prevents SCF-dependent differentiation of mast cells in vitro and results in decreased mast cell numbers in vivo.30 Furthermore, human mast cells are not generated by IL-3 alone in vitro. Therefore, the general assumption is that human mast cells require SCF and KIT signaling for their survival, proliferation, and maturation.13,14
In the present study, we assessed the validity of the prevailing consensus that SCF and KIT signaling are critically needed for human mast cell development. We demonstrate that disrupting KIT signaling does not affect the frequency of mast cell progenitors in vivo and that SCF and KIT signaling are dispensable for the survival, proliferation, and maturation of mast cell progenitors in vitro. Thus, although SCF and KIT signaling stimulates the proliferation and differentiation of human mast cells, their importance in the context of mast cell progenitors has been overestimated.
Patients, materials, and methods
Patients and healthy subjects
The local ethics committees approved the experiments involving human subjects, when applicable, and the participants provided informed consent. Ethics approval was not needed for anonymous collection of buffy coats, in accordance with Swedish legislation, because they cannot be traced to a specific person. Patients with chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) were under continuous treatment with imatinib. The imatinib dose was 300 to 800 mg daily, and the treatment time ranged between 1 and 130 months at the time of analysis. The presence of the D816V mutation in the KIT gene was the inclusion criterion for the systemic mastocytosis patients. One patient with the D816V mutation was pending diagnosis confirmation.
Isolation and culture of human hematopoietic progenitors
Peripheral blood from patients and control subjects was collected into EDTA tubes, and leukocytes were purified using BD Pharm Lyse lysing solution (BD Biosciences, Franklin Lakes, NJ). Hematopoietic progenitors were isolated from the buffy coats of healthy donors. First, mononuclear cells were purified using Ficoll-Paque PLUS (GE Healthcare, Chicago, IL), and this was followed by enrichment of CD34+ progenitors with a CD34 MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). In some experiments, the purified CD34+ progenitors were labeled with CellTrace Far Red (Thermo Fisher Scientific, Waltham, MA) before culture. Pure mast cells progenitors were isolated with a CD117 MicroBead Kit and then subjected to fluorescence-activated cell sorting (FACS) of the CD34+CD117int/hiFcεRI+ cells.
Isolated CD34+ progenitors or mast cell progenitors were cultured with complete Gibco StemPro-34 serum-free medium (Thermo Fisher Scientific) with 2 mM l-glutamine (Sigma-Aldrich, St. Louis, MO), 100 U/mL penicillin (Sigma-Aldrich), and 0.1 mg/mL streptomycin (Sigma-Aldrich). The medium was supplemented with the recombinant human cytokines IL-3 (10 ng/mL; PeproTech, Rocky Hill, NJ), IL-6 (10 ng/mL; PeproTech), and SCF (100 ng/mL, r-metHuSCF; Swedish Orphan Biovitrum, Stockholm, Sweden) individually or in combination, as indicated in the figure legends. In some experiments, 10 μM imatinib mesylate (Sigma-Aldrich) dissolved in dimethyl sulfoxide or vehicle only was added to the culture medium. Polyclonal goat anti-human SCF or polyclonal goat IgG (5 μg/mL; both from PeproTech) was added to the culture medium in some experiments. Enzyme cytochemical staining with the Z-Gly-Pro-Arg-4-Methoxy-β-naphthylamide (Bachem, Bubendorf, Switzerland) substrate was used to assess trypsinlike activity.31
Flow cytometric analyses and cell sorting
Fluorophore-conjugated antibodies against CD4 (RPA-T4), CD8 (RPA-T8), CD14 (M5E2), CD19 (HIB19), CD34 (581), CD45 (HI30), CD117 (104D2 and A3E6E2), CD123/IL-3R (6H6), integrin β7 (FIB504), and FcεRI (CRA1) were used to stain the cells. The use of the live/dead marker Fixable Viability Stain 450 (BD Biosciences) is indicated in the figure legends. The antibodies were from BD Biosciences, Biolegend (San Diego, CA), Miltenyi Biotec, Dako (Agilent Technologies, Santa Clara, CA), and eBioscience (San Diego, CA). FACS was performed with a FACSAria I instrument. Flow cytometric analyses were performed with FACSCanto II, FACSAria I, FACSAria III, or LSRFortessa instruments. The mean number of CD45+ singlet cells recorded per sample was 7.8 million when determining the frequency of mast cell progenitors and mast cells (range: 0.86-15.8 million). The data analysis was performed using FlowJo software (TreeStar, Ashland, OR). Gates were set according to the fluorescence minus 1 controls, the fluorescence minus 1 controls with isotype control antibody staining, or internal controls.
Statistics
Statistics were calculated using Prism software (GraphPad Software, La Jolla, CA). Unpaired 2-tailed Student t tests were used when 2 groups were compared, and a 1-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used when 3 or more groups were compared. The data involving patients and control subjects were log-transformed before the statistical analyses. Differences were considered significant when the P < .05.
Results
Mast cell progenitors survive imatinib treatment in vivo
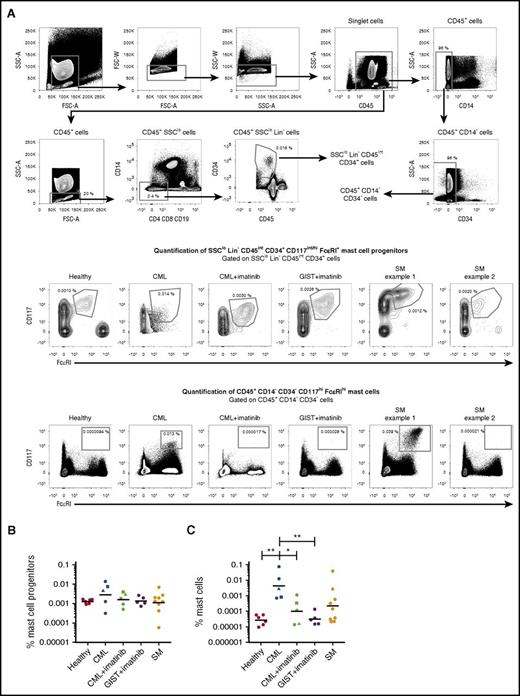
The frequencies of peripheral blood mast cell progenitors and mast cells were analyzed in healthy control subjects and in patients with dysfunctional KIT signaling. Circulating mast cell progenitors, identified as CD45intCD34+SSCloLin−CD117int/hiFcεRI+ cells, constituted 0.0013% (geometric mean) of the CD45+ cell population in the blood of healthy control subjects (Figure 1A-B). The importance of KIT signaling for the presence of blood mast cell progenitors was studied through 3 different approaches. First, CML patients treated with the tyrosine kinase inhibitor imatinib, which disrupts KIT signaling, were studied. Imatinib had efficiently restored the leukocyte concentration in the blood of CML patients (supplemental Table 1, available on the Blood Web site). Nonetheless, the imatinib-treated CML patients had mast cell progenitor frequencies similar to those in healthy subjects (Figure 1A-B). Second, patients undergoing imatinib treatment of GIST, a nonhematological disease, were also evaluated. Both these patients and healthy subjects had similar numbers of mast cell progenitors (Figure 1A-B). Third, patients diagnosed with systemic mastocytosis carrying the D816V KIT mutation, which causes KIT signaling to be constitutively active, were studied. These patients had the same order of magnitude of mast cell progenitors as healthy subjects (Figure 1A-B). Thus, dysfunctional KIT signaling does not affect the frequency of circulating mast cell progenitors in vivo.
Imatinib treatment does not affect the frequency of blood mast cell progenitors in vivo. (A) Peripheral blood mast cell progenitors and mast cells were gated as SSCloLin−CD45intCD34+CD117int/hiFcεRI+ cells and CD45+CD14−CD34−CD117hiFcεRIhi cells, respectively. The percentages indicate the frequency of the gated cells normalized to the number of CD45+ singlet cells. The frequencies of mast cell progenitors (B) and mast cells (C) from the CD45+ cell population in healthy controls (n = 6), patients with CML (n = 5), patients with CML under treatment with imatinib (n = 5), patients with GIST under treatment with imatinib (n = 5), and patients with systemic mastocytosis (SM) carrying the D816V KIT mutation (n = 9) are shown. The lines represent the geometric means. The triangles and squares represent the same patients before and during imatinib treatment. One-way ANOVA with Tukey’s multiple comparisons test on log-transformed data. *Adjusted P < .05; **adjusted P < .01. FSC, forward scatter; SSC, side scatter.
Imatinib treatment does not affect the frequency of blood mast cell progenitors in vivo. (A) Peripheral blood mast cell progenitors and mast cells were gated as SSCloLin−CD45intCD34+CD117int/hiFcεRI+ cells and CD45+CD14−CD34−CD117hiFcεRIhi cells, respectively. The percentages indicate the frequency of the gated cells normalized to the number of CD45+ singlet cells. The frequencies of mast cell progenitors (B) and mast cells (C) from the CD45+ cell population in healthy controls (n = 6), patients with CML (n = 5), patients with CML under treatment with imatinib (n = 5), patients with GIST under treatment with imatinib (n = 5), and patients with systemic mastocytosis (SM) carrying the D816V KIT mutation (n = 9) are shown. The lines represent the geometric means. The triangles and squares represent the same patients before and during imatinib treatment. One-way ANOVA with Tukey’s multiple comparisons test on log-transformed data. *Adjusted P < .05; **adjusted P < .01. FSC, forward scatter; SSC, side scatter.
Virtually no CD45+CD14−CD34−CD117hiFcεRIhi mast cells were found in the peripheral blood of healthy control subjects (Figure 1A,C). In contrast, peripheral blood mast cells were found in a subset of systemic mastocytosis patients with the D816V KIT mutation (Figure 1A,C), as previously reported.17 Notably, peripheral blood mast cells were also detected in patients with CML at diagnosis (Figure 1A,C). These mast cells were decreased in frequency in CML patients undergoing treatment with imatinib (Figure 1A,C). Thus, circulating mast cells, but not mast cell progenitors, are sensitive to imatinib in patients with CML.
Mast cell progenitors survive without SCF in vitro
We next investigated whether human mast cell progenitors could survive without SCF in vitro. CD34+ progenitors were purified from human peripheral blood and cultured under different conditions: without cytokines; with IL-3 and IL-6; or with IL-3, IL-6, and SCF for 3, 5, and 7 days. A population of CD34+CD117int/hiFcεRI+ mast cell progenitors was identified before culturing (Figure 2A-B). Culturing of the CD34+ progenitors with IL-3 and IL-6 promoted the survival of the CD117hiFcεRI+ cells, whereas this population was lost when the cells were cultured without cytokines (Figure 2C-D). The downregulation of CD34 at days 5 and 7 in the IL-3 and IL-6 condition indicated that the CD117hiFcεRI+ cells had matured (Figure 2E). Thus, CD117hiFcεRI+ cells cultured for 5 and 7 days will be referred to as pre–mast cells henceforth. No clear pre–mast cell population was distinguished when the CD34+ progenitors were cultured with IL-3, IL-6, and SCF, probably because of the SCF-dependent internalization of KIT (Figure 2C-D). Indeed, the CD34+ cells cultured with IL-3, IL-6, and SCF already had lower KIT expression after 3 days than was found in the corresponding cells cultured with IL-3 and IL-6 or without cytokines (Figure 2F-G). Altogether, the cytokines IL-3 and IL-6 are sufficient to promote the survival of mast cell progenitors in vitro.
SCF is dispensable for mast cell progenitor survival in vitro. CD34+ progenitors were purified from buffy coats from healthy donors and analyzed with flow cytometry. (A-B) The representative gating strategy and the quantification of CD34+CD117int/hiFcεRI+ mast cell progenitors are shown (n = 9). The representative example in panel A is indicated with an open circle. The line in panel B represents the geometric mean. (C-G) CD34-enriched cells were cultured and analyzed by flow cytometry. (D) The fraction of CD117hiFcεRI+ pre–mast cells from panel C was normalized to the combined IL-3 and IL-6 condition for each buffy coat, and the results from 3 buffy coats were pooled. The bars represent the means ± standard error of the mean (SEM). (E) CD34+ progenitors were cultured with IL-3 and IL-6, and the fractions of CD34-expressing cells out of the CD117hiFcεRI+ mast cell population (days 3-7) are shown. The CD34 expression of CD117int/hiFcεRI+ cells is shown as the day 0 control. The bars represent the means ± SEM of 3 buffy coats. (F) The CD117 expression of CD34+ cells was analyzed on day 3. The red histogram indicates the cells cultured without cytokines. The blue line indicates the cells cultured with IL-3 and IL-6. The dashed line indicates the cells cultured with IL-3, IL-6, and SCF. One representative experiment out of 3 is shown. (G) The CD117 expression on CD34+ cells at day 3, shown in panel F, was quantified by calculation of the median fluorescence intensity. The expression level of CD117 is shown as a percentage of the IL-3 and IL-6 condition. The bars represent the means ± SEM of 3 buffy coats. (H-J) CD34+ cells were enriched from buffy coats, cultured, and analyzed by flow cytometry. The medium was supplemented with polyclonal goat IgG or anti-SCF neutralizing antibodies as indicated. (J) The integrin β7 expression was analyzed by flow cytometry before and after culture with IL-3, IL-6, and anti-SCF. The bars in panels I and J represent the means ± SEM of 3 buffy coats. Live singlet cells are shown in the flow cytometric graphs. The statistical analyses in panels D, E, G, and I were performed using 1-way ANOVA with Tukey’s multiple comparisons test. **Adjusted P < .01; ****adjusted P < .0001; ns = not significant. The unpaired 2-tailed Student t test was used for the statistical analysis in panel J. ****P < .0001.
SCF is dispensable for mast cell progenitor survival in vitro. CD34+ progenitors were purified from buffy coats from healthy donors and analyzed with flow cytometry. (A-B) The representative gating strategy and the quantification of CD34+CD117int/hiFcεRI+ mast cell progenitors are shown (n = 9). The representative example in panel A is indicated with an open circle. The line in panel B represents the geometric mean. (C-G) CD34-enriched cells were cultured and analyzed by flow cytometry. (D) The fraction of CD117hiFcεRI+ pre–mast cells from panel C was normalized to the combined IL-3 and IL-6 condition for each buffy coat, and the results from 3 buffy coats were pooled. The bars represent the means ± standard error of the mean (SEM). (E) CD34+ progenitors were cultured with IL-3 and IL-6, and the fractions of CD34-expressing cells out of the CD117hiFcεRI+ mast cell population (days 3-7) are shown. The CD34 expression of CD117int/hiFcεRI+ cells is shown as the day 0 control. The bars represent the means ± SEM of 3 buffy coats. (F) The CD117 expression of CD34+ cells was analyzed on day 3. The red histogram indicates the cells cultured without cytokines. The blue line indicates the cells cultured with IL-3 and IL-6. The dashed line indicates the cells cultured with IL-3, IL-6, and SCF. One representative experiment out of 3 is shown. (G) The CD117 expression on CD34+ cells at day 3, shown in panel F, was quantified by calculation of the median fluorescence intensity. The expression level of CD117 is shown as a percentage of the IL-3 and IL-6 condition. The bars represent the means ± SEM of 3 buffy coats. (H-J) CD34+ cells were enriched from buffy coats, cultured, and analyzed by flow cytometry. The medium was supplemented with polyclonal goat IgG or anti-SCF neutralizing antibodies as indicated. (J) The integrin β7 expression was analyzed by flow cytometry before and after culture with IL-3, IL-6, and anti-SCF. The bars in panels I and J represent the means ± SEM of 3 buffy coats. Live singlet cells are shown in the flow cytometric graphs. The statistical analyses in panels D, E, G, and I were performed using 1-way ANOVA with Tukey’s multiple comparisons test. **Adjusted P < .01; ****adjusted P < .0001; ns = not significant. The unpaired 2-tailed Student t test was used for the statistical analysis in panel J. ****P < .0001.
The CD34+ progenitors were cultured with goat IgG control antibodies or anti-SCF neutralizing antibodies for 5 days to study whether mast cell progenitor survival was dependent on endogenously produced SCF. As expected, the pre–mast cell population was present when the CD34+ progenitors were cultured with IL-3, IL-6, and goat IgG control antibodies (Figure 2H-I). No clear pre–mast cell population was observed when SCF was added to the same culture condition (Figure 2H-I), again likely because of KIT internalization. Notably, pre–mast cells were present in cultures with IL-3, IL-6, and anti-SCF, with or without SCF (Figure 2H-I). These findings suggest that anti-SCF antibodies prevent SCF-dependent downregulation of KIT. Importantly, the results clearly demonstrate that mast cell progenitors survive in the absence of SCF.
Integrin β7 is a phenotypic marker of mouse mast cell progenitors, and the surface expression of this integrin is downregulated on maturation, both in vivo and in vitro.32-35 Integrin β7 was downregulated when the human mast cell progenitors were cultured in the absence of SCF (Figure 2J), consistent with mouse mast cell progenitor maturation.
Mast cell progenitors survive without KIT signaling in vitro
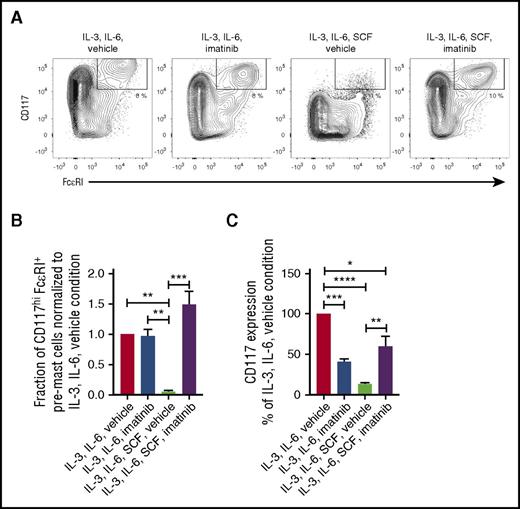
CD34+ progenitors were cultured with or without imatinib for 5 days to determine whether KIT signaling is critical for mast cell progenitor survival in vitro. Imatinib treatment did not affect the survival of mast cell progenitors cultured with IL-3 and IL-6 (Figure 3A-B). In the absence of imatinib, SCF treatment resulted in the downregulation of KIT surface expression in cultures with IL-3 and IL-6 (Figure 3A,C). However, KIT downregulation was inhibited when imatinib was added to the cultures with IL-3, IL-6, and SCF (Figure 3A,C). Notably, the cultures treated with IL-3, IL-6, SCF, and imatinib had a frequency of CD117hiFcεRI+ cells, similarly to cells treated with only IL-3 and IL-6 (Figure 3A-B). Thus, mast cell progenitors do not need KIT signaling for survival in vitro.
KIT signaling is dispensable for mast cell progenitor survival. (A-C) CD34+ progenitors were enriched from buffy coats, cultured for 5 days, and analyzed by flow cytometry. The medium was supplemented with imatinib dissolved in dimethyl sulfoxide where indicated. Vehicle indicates that the medium was supplemented with dimethyl sulfoxide only. (B) The fraction of CD117hiFcεRI+ pre–mast cells from panel A was normalized to the combined IL-3, IL-6, and vehicle condition for each buffy coat (n = 3). The bars represent the means ± SEM. (C) The CD117 expression on CD34+ cells from day 5 was quantified by calculation of the median fluorescence intensity. The expression level of CD117 is shown as a percentage of the IL-3, IL-6, and vehicle condition. The bars represent the means ± SEM of 3 buffy coats. Live singlet cells are shown in the flow cytometric graphs. All statistical analyses were performed using 1-way ANOVA with Tukey’s multiple comparisons test. *Adjusted P < .05; **adjusted P < .01; ***adjusted P < .001; ****adjusted P < .0001.
KIT signaling is dispensable for mast cell progenitor survival. (A-C) CD34+ progenitors were enriched from buffy coats, cultured for 5 days, and analyzed by flow cytometry. The medium was supplemented with imatinib dissolved in dimethyl sulfoxide where indicated. Vehicle indicates that the medium was supplemented with dimethyl sulfoxide only. (B) The fraction of CD117hiFcεRI+ pre–mast cells from panel A was normalized to the combined IL-3, IL-6, and vehicle condition for each buffy coat (n = 3). The bars represent the means ± SEM. (C) The CD117 expression on CD34+ cells from day 5 was quantified by calculation of the median fluorescence intensity. The expression level of CD117 is shown as a percentage of the IL-3, IL-6, and vehicle condition. The bars represent the means ± SEM of 3 buffy coats. Live singlet cells are shown in the flow cytometric graphs. All statistical analyses were performed using 1-way ANOVA with Tukey’s multiple comparisons test. *Adjusted P < .05; **adjusted P < .01; ***adjusted P < .001; ****adjusted P < .0001.
Mast cell progenitors mature without SCF in vitro
Cell surface CD34 decreases and is subsequently lost during human mast cell maturation.36 Mast cell progenitors exhibited downregulated CD34 expression during culture even without SCF (Figure 2E). Nonetheless, the finding that mast cell progenitors were able to mature without SCF was evaluated further. The size and granularity of the mast cell progenitors and pre–mast cells were measured by using the forward and side scatter parameters, respectively. The IL-3- and IL-6-cultured pre–mast cells were larger and had higher granularity than did the primary mast cell progenitors (Figure 4A-B). Comparable results were obtained when the progenitors were cultured with IL-3, IL-6, and anti-SCF, a culture condition in which any endogenously produced SCF would be neutralized (supplemental Figure 1). Altogether, the findings indicate that the mast cell progenitors matured without SCF.
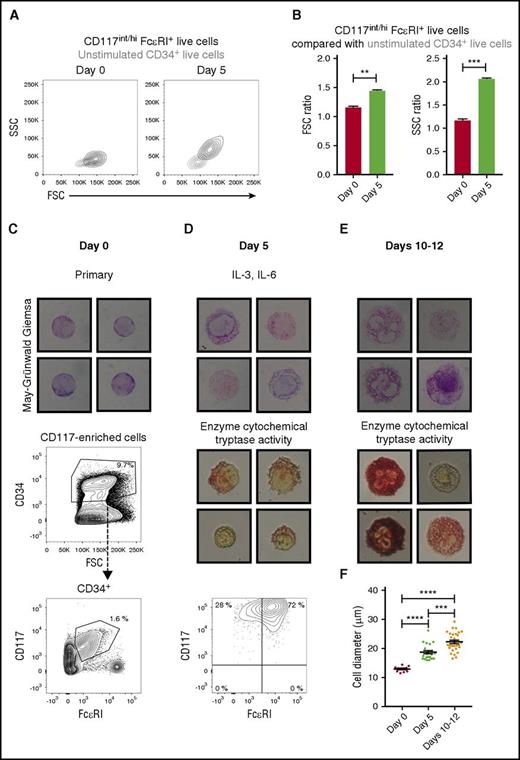
Mast cell progenitors mature in the absence of SCF. (A) The CD34-enriched progenitors were purified from buffy coats and cultured with IL-3 and IL-6. The FSC and SSC parameters of the primary mast cell progenitors (CD117int/hiFcεRI+ cells, left) and the cultured pre–mast cells (CD117hiFcεRI+ cells, right) are shown in black height curves. The gray height curves indicate unstimulated CD34+ cells. One representative buffy coat out of 3 is shown. (B) The mean fluorescence intensities of the FSC and SSC parameters were calculated for each population described in panel A. The ratio between the CD117int/hiFcεRI+ cells and the unstimulated CD34+ cells was then calculated for each time point independently, as the photomultiplier tube voltages of those parameters were adjusted between day 0 and 5 to visualize the cells on scale. The results were pooled from 3 buffy coats. The bars represent the means ± SEM. Unpaired 2-tailed Student t tests. **P < .01; ***P < .001. (C) CD117+ cells were enriched from buffy coats, and CD34+CD117int/hiFcεRI+ cells were isolated with FACS. The isolated CD34+CD117int/hiFcεRI+ cells were stained with May-Grünwald Giemsa. (D) Sorted CD34+CD117int/hiFcεRI+ cells were cultured with IL-3 and IL-6 for 5 days. The cultured cells were analyzed with May-Grünwald Giemsa staining, an enzyme cytochemical staining for trypsinlike activity as a measure of tryptase, and flow cytometry. Live singlet cells are shown in the flow cytometric graph. (E) Sorted CD34+CD117int/hiFcεRI+ cells were first cultured with IL-3 and IL-6 for 5 days and then cultured with SCF and IL-6 for an additional 5 to 7 days. The cultured cells were analyzed with May-Grünwald Giemsa staining and an enzyme cytochemical staining for trypsinlike activity as a measure of tryptase. (F) The largest cell diameter of the primary mast cell progenitors, pre–mast cells, and mast cells was measured using ImageJ (NIH, Bethesda, MD). Each dot in the graph corresponds to 1 cell. The means ± SEM are shown. One-way ANOVA with Tukey’s multiple comparisons test. ***Adjusted P < .001; ****adjusted P < .0001. Images were captured using the Eclipse E400 microscope, the Digital Camera DXM1200, and ACT-1 software (Nikon, Tokyo, Japan). The width of the May-Grünwald Giemsa photos corresponds to 28 μm. The width of the enzyme cytochemical staining photos corresponds to 35 μm. The cultures at day 5 and days 10 to 12 were performed for 2 buffy coats with similar results. The day 0 May-Grünwald Giemsa staining was performed for 1 buffy coat.
Mast cell progenitors mature in the absence of SCF. (A) The CD34-enriched progenitors were purified from buffy coats and cultured with IL-3 and IL-6. The FSC and SSC parameters of the primary mast cell progenitors (CD117int/hiFcεRI+ cells, left) and the cultured pre–mast cells (CD117hiFcεRI+ cells, right) are shown in black height curves. The gray height curves indicate unstimulated CD34+ cells. One representative buffy coat out of 3 is shown. (B) The mean fluorescence intensities of the FSC and SSC parameters were calculated for each population described in panel A. The ratio between the CD117int/hiFcεRI+ cells and the unstimulated CD34+ cells was then calculated for each time point independently, as the photomultiplier tube voltages of those parameters were adjusted between day 0 and 5 to visualize the cells on scale. The results were pooled from 3 buffy coats. The bars represent the means ± SEM. Unpaired 2-tailed Student t tests. **P < .01; ***P < .001. (C) CD117+ cells were enriched from buffy coats, and CD34+CD117int/hiFcεRI+ cells were isolated with FACS. The isolated CD34+CD117int/hiFcεRI+ cells were stained with May-Grünwald Giemsa. (D) Sorted CD34+CD117int/hiFcεRI+ cells were cultured with IL-3 and IL-6 for 5 days. The cultured cells were analyzed with May-Grünwald Giemsa staining, an enzyme cytochemical staining for trypsinlike activity as a measure of tryptase, and flow cytometry. Live singlet cells are shown in the flow cytometric graph. (E) Sorted CD34+CD117int/hiFcεRI+ cells were first cultured with IL-3 and IL-6 for 5 days and then cultured with SCF and IL-6 for an additional 5 to 7 days. The cultured cells were analyzed with May-Grünwald Giemsa staining and an enzyme cytochemical staining for trypsinlike activity as a measure of tryptase. (F) The largest cell diameter of the primary mast cell progenitors, pre–mast cells, and mast cells was measured using ImageJ (NIH, Bethesda, MD). Each dot in the graph corresponds to 1 cell. The means ± SEM are shown. One-way ANOVA with Tukey’s multiple comparisons test. ***Adjusted P < .001; ****adjusted P < .0001. Images were captured using the Eclipse E400 microscope, the Digital Camera DXM1200, and ACT-1 software (Nikon, Tokyo, Japan). The width of the May-Grünwald Giemsa photos corresponds to 28 μm. The width of the enzyme cytochemical staining photos corresponds to 35 μm. The cultures at day 5 and days 10 to 12 were performed for 2 buffy coats with similar results. The day 0 May-Grünwald Giemsa staining was performed for 1 buffy coat.
The CD34+CD117int/hiFcεRI+ mast cell progenitors were next isolated through FACS (Figure 4C). These cells had an immature phenotype, in which the nuclei were large in relation to the cytoplasm, and the cells had no or few granules (Figure 4C). This phenotype is consistent with previous observations for mast cell progenitors.15 The pure CD34+CD117int/hiFcεRI+ mast cell progenitors were cultured for 5 days in medium containing IL-3 and IL-6. As expected, the cultured cells retained high CD117 expression, and the majority still expressed FcεRI (Figure 4D). The pre–mast cells were larger than the primary mast cell progenitors, and they had developed granules, some of which were metachromatic (Figure 4D,F). Some cells stained faintly for trypsinlike activity, thus indicating that the cells had begun to produce tryptase (Figure 4D).
To confirm that the primary CD34+CD117int/hiFcεRI+ progenitors and the pre–mast cells could form mast cells, the IL-3- and IL-6-containing medium was replaced on day 5 with medium containing SCF and IL-6 (Figure 4E). The cells increased in size, rapidly formed numerous metachromatic granules, and stained strongly for trypsinlike activity after an additional 5 to 7 days in culture (Figure 4E-F). Together, these results indicate that CD34+CD117int/hiFcεRI+ progenitors can mature into pre–mast cells in the absence of SCF.
Mast cell progenitors proliferate without SCF in vitro
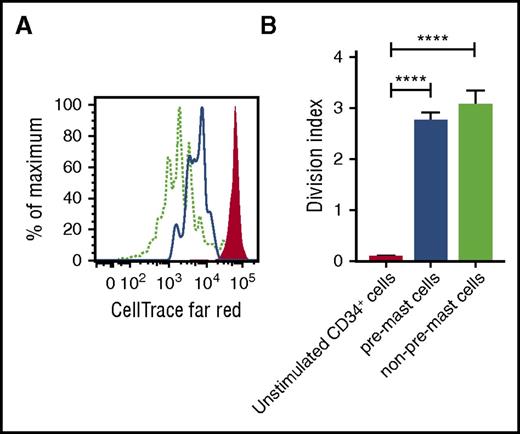
Next, we tested whether CD34+ mast cell progenitors could proliferate without SCF. CD34+ cells were labeled with the cell proliferation dye CellTrace Far Red, which enables the tracking of the number of cell divisions that progenitors undergo. The CD34+ progenitors were cultured for 5 days with IL-3 and IL-6. CD117hiFcεRI+ pre–mast cells were gated, and the number of cell divisions was analyzed. Cells falling outside the CD117hiFcεRI+ gate, constituting mixed cell types, were used as a positive control for the proliferation assay. Unstimulated CellTrace-labeled CD34+ cells incubated at 2°C–8°C for 5 days were used as a negative control. The CD117int/hi FcεRI+ progenitors divided when cultured with IL-3 and IL-6, with a mean division index of 2.8 (Figure 5A-B). Thus, mast cell progenitors can proliferate in the absence of SCF.
Mast cell progenitors proliferate in the absence of SCF. CD34-enriched cells purified from buffy coats were stained with CellTrace Far Red. (A) The cells were cultured with IL-3 and IL-6 for 5 days and analyzed by flow cytometry. Live singlets were gated. The red-filled histogram represents unstimulated CD34+ cells, the blue line indicates CD117hiFcεRI+ pre–mast cells, and the dotted light green line indicates cells that did not fall into the CD117hiFcεRI+ pre–mast cell gate. (B) Cell proliferation was analyzed using the proliferation platform in FlowJo. The model was adjusted to 8 peaks. The non–pre-mast-cell population was used as a positive control for cell division, and the cell generation gates were calculated accordingly. The undivided peak was set according to the unstimulated sample, which was stored at 2°C–8°C throughout the culture period. The division index corresponds to the mean number of cell divisions undergone by the cells in the original culture. The bars represent the means ± SEM of 3 buffy coats. One-way ANOVA with Tukey’s multiple comparisons test. ****Adjusted P < .0001.
Mast cell progenitors proliferate in the absence of SCF. CD34-enriched cells purified from buffy coats were stained with CellTrace Far Red. (A) The cells were cultured with IL-3 and IL-6 for 5 days and analyzed by flow cytometry. Live singlets were gated. The red-filled histogram represents unstimulated CD34+ cells, the blue line indicates CD117hiFcεRI+ pre–mast cells, and the dotted light green line indicates cells that did not fall into the CD117hiFcεRI+ pre–mast cell gate. (B) Cell proliferation was analyzed using the proliferation platform in FlowJo. The model was adjusted to 8 peaks. The non–pre-mast-cell population was used as a positive control for cell division, and the cell generation gates were calculated accordingly. The undivided peak was set according to the unstimulated sample, which was stored at 2°C–8°C throughout the culture period. The division index corresponds to the mean number of cell divisions undergone by the cells in the original culture. The bars represent the means ± SEM of 3 buffy coats. One-way ANOVA with Tukey’s multiple comparisons test. ****Adjusted P < .0001.
IL-3 is sufficient to promote the survival of mast cell progenitors in vitro
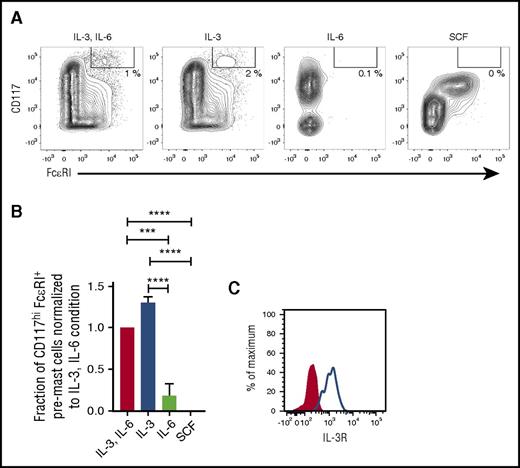
CD34+ progenitors were cultured with IL-3 and IL-6 in combination or with each cytokine alone for 5 days to determine whether only one of the cytokines might be sufficient to promote the survival of mast cell progenitors. IL-3 alone promoted the survival of mast cell progenitors, whereas IL-6 alone was unable to promote the survival of mast cell progenitors (Figure 6A-B). Primary mast cell progenitors express the receptor for IL-3, as verified by flow cytometry (Figure 6C). Altogether, IL-3 is sufficient for mast cell progenitor survival in vitro, whereas SCF and IL-6 are dispensable.
IL-3 is sufficient for mast cell progenitor survival in vitro. CD34+ progenitors were enriched from buffy coats and cultured for 5 days with the cytokines indicated in the figure. (A) The cultured cells were analyzed with flow cytometry, and the frequency of CD117hiFcεRI+ pre–mast cells as a fraction of live singlet cells was quantified. One representative experiment out of 3 is shown. (B) The frequency of CD117hiFcεRI+ pre–mast cells as a fraction of live singlet cells was normalized to the combined IL-3 and IL-6 condition for each buffy coat, and the results from 3 buffy coats were pooled. The bars represent the means ± SEM of each group. One-way ANOVA with Tukey’s multiple comparisons test. ***Adjusted P < .001; ****adjusted P < .0001. (C) The IL-3 receptor (IL-3R) expression on the SSCloCD14−CD34+CD117int/hiFcεRI+ blood mast cell progenitors is shown. The red-filled histogram indicates the fluorescence minus 1 control, and the blue line indicates the sample. The analysis was performed on cells from 2 blood donors and yielded similar results.
IL-3 is sufficient for mast cell progenitor survival in vitro. CD34+ progenitors were enriched from buffy coats and cultured for 5 days with the cytokines indicated in the figure. (A) The cultured cells were analyzed with flow cytometry, and the frequency of CD117hiFcεRI+ pre–mast cells as a fraction of live singlet cells was quantified. One representative experiment out of 3 is shown. (B) The frequency of CD117hiFcεRI+ pre–mast cells as a fraction of live singlet cells was normalized to the combined IL-3 and IL-6 condition for each buffy coat, and the results from 3 buffy coats were pooled. The bars represent the means ± SEM of each group. One-way ANOVA with Tukey’s multiple comparisons test. ***Adjusted P < .001; ****adjusted P < .0001. (C) The IL-3 receptor (IL-3R) expression on the SSCloCD14−CD34+CD117int/hiFcεRI+ blood mast cell progenitors is shown. The red-filled histogram indicates the fluorescence minus 1 control, and the blue line indicates the sample. The analysis was performed on cells from 2 blood donors and yielded similar results.
Discussion
This study demonstrates that peripheral blood mast cell progenitors can survive the inhibition of KIT signaling caused by imatinib treatment in patients with CML and GIST. Interestingly, a population of CD45+CD14−CD34−CD117hiFcεRIhi cells was found in the peripheral blood of all patients with newly diagnosed CML and in a subset of patients with systemic mastocytosis, but not in healthy controls. CD34−CD117hiFcεRIhi mast cells in the peripheral blood have previously been reported in patients with advanced forms of systemic mastocytosis.17 Our current findings show that the circulating mast cells in the CML patients were almost completely depleted by imatinib treatment. The depletion of mast cells in imatinib-treated patients was consistent with a previous report that mast cell numbers decrease in bone marrow during imatinib treatment.30 Thus, mast cells, but not mast cell progenitors, in CML patients are sensitive to imatinib.
IL-3 is commonly supplemented in IL-6- and SCF-containing medium in the early phase of in vitro cultures to generate human mast cells. We verified that primary mast cell progenitors expressed IL-3R and that IL-3 alone promoted the survival of mast cell progenitors. The survival-promoting effects of IL-3 are probably limited to mast cell progenitors, because (mature) mast cells from several tissues, such as the skin, lung, uterus, and tonsils, lack IL-3R expression.37,38 However, IL-3R expression has been reported in intestinal mast cells.39
Many studies have demonstrated that IL-3 is not sufficient for the full maturation of mast cells.1-3 In fact, prolonged culture with IL-3 inhibits the SCF-induced generation and maturation of mast cells.40,41 Our findings show that mast cell progenitors, which virtually lack granules, can form granulated pre–mast cells in the absence of SCF and the presence of IL-3 and IL-6. When IL-3 was removed and replaced with SCF, the pre–mast cells rapidly matured.
The requirement of SCF and KIT signaling for mast cell progenitor survival was investigated further in vitro. The mast cell progenitors were cultured with imatinib to mimic the in vivo setting in which subjects are treated with imatinib. Imatinib at a concentration of 1 μM has been reported to almost completely inhibit mast cell generation in vitro.30 Moreover, the survival of mast cells in vitro decreased by 70% within 3 days after treatment with the same concentration of imatinib.42 At a concentration of 1 μM, imatinib also prevents the SCF-dependent internalization of CD117.43 In the current study, we used 10 μM imatinib to inhibit KIT signaling in vitro. This treatment inhibited the SCF-dependent internalization of CD117, thus suggesting that the treatment was efficient. However, the treatment did not affect the frequency of pre–mast cells, thus indicating that mast cell progenitors do not require KIT signaling for survival. The CD34-enriched cells from the peripheral blood were seeded at a low concentration (20 000 cells/mL) to avoid the confounding effects of endogenously produced cytokines. Nevertheless, we ruled out the possibilities that the mast cell progenitor survival and maturation were dependent on endogenously produced SCF by showing that pre–mast cells could be generated in the presence of neutralizing anti-SCF antibodies.
Notably, early studies have demonstrated that human bone marrow progenitors cultured with IL-3 alone give rise to low frequencies of mast cells in vitro.44,45 The bone marrow cells cultured in these studies were from patients under evaluation for or with known mastocytosis. Mastocytosis is typically associated with the D816V mutation in the gene encoding KIT, which results in the constitutive activation of the receptor. Mast cells can be generated from those patients even without cytokine stimulation.46 Our findings show that IL-3 and IL-6 are sufficient for mast cell progenitors to survive, proliferate, and mature into pre–mast cells in healthy subjects. The source of the mast cell progenitors for the in vitro experiments in the present study was peripheral blood. Whether SCF is strictly required for mast cell progenitor survival, proliferation, and maturation during prenatal development was not determined.
In addition to IL-3 and IL-6, other growth factors, such as granulocyte-macrophage colony-stimulating factor, IL-9, and thrombopoietin, can also modulate the SCF-dependent formation of human mast cells in vitro.41,47-50 The effects of these and other growth factors on mast cell progenitors have not been studied. However, the finding that mast cell progenitors can survive in vivo in imatinib-treated patients indicates that KIT signaling is dispensable for mast cell progenitor survival. Imatinib targets several tyrosine kinases, and it is therefore probable that mast cell progenitors do not require signaling through receptors such as CSF-1R and PDGFR for their survival. It is possible that several growth factors and receptors may act redundantly in vivo in the absence of KIT signaling, but our findings show that IL-3 is sufficient for mast cell progenitor survival in vitro. The data presented here and elsewhere also indicate that SCF is nonetheless important for the full maturation of the mast cell lineage in vitro.41
Mast cells play a crucial role in the pathogenesis of diseases, such as mastocytosis, allergies, asthma, and other chronic inflammatory disorders.51,52 Targeting KIT signaling has been proposed as a strategy to decrease the mast cell burden in D816V-negative patients with mastocytosis. Our results show that even though the number of mast cells can be decreased with imatinib treatment, the pool of mast cell progenitors remains. Consequently, other strategies beyond the inhibition of KIT signaling are needed to deplete both the mast cell progenitor population and the mast cells. Our data show that IL-3R is a potential target for depleting mast cell progenitors. This finding is particularly interesting as it relates to patients with systemic mastocytosis with IL-3R expression on neoplastic mast cells.53 SL-401, a fusion protein containing a diphtheria toxin domain and an IL-3 domain, is currently in clinical trials for the treatment of systemic mastocytosis (www.clinicaltrials.gov; #NCT02268253). Perhaps SL-401 will target both neoplastic mast cells and mast cell progenitors. The effects of SL-401 and other therapies targeting IL-3R in systemic mastocytosis patients require further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Swedish Orphan Biovitrum for the generous gift of recombinant SCF.
This work was supported by the Swedish Research Council, the Swedish Cancer Society, the Cancer and Allergy Foundation, Stockholm Cancer Society (Radiumhemmets Forskningsfonder), Tore Nilson’s Foundation for Medical Research, the Ollie and Elof Ericsson Foundation, Hans von Kantzow’s Foundation, and the Karolinska Institutet.
Authorship
Contribution: J.S.D., M.E., and G.N. designed the experiments; J.S.D. and M.E. performed the experiments involving the buffy coats; J.S.D., M.E., L.L., and J.G. performed the experiments involving the patients and control samples; H.H., U.O.-S., and J.S.U. diagnosed the patients and provided the patient samples; R.-M.A. characterized the patients; J.S.D., M.E., and G.N. analyzed the data; J.S.D. and G.N. wrote the original draft; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gunnar Nilsson, Department of Medicine, Karolinska Institutet, SE-171 76 Stockholm, Sweden; e-mail: gunnar.p.nilsson@ki.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal