Key Points
SCHOLAR-1 is the first patient-level analysis of outcomes of refractory DLBCL from 2 large randomized trials and 2 academic databases.
SCHOLAR-1 demonstrated poor outcomes in patients with refractory DLBCL, supporting a need for more effective therapies for these patients.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma. Although 5-year survival rates in the first-line setting range from 60% to 70%, up to 50% of patients become refractory to or relapse after treatment. Published analyses of large-scale outcome data from patients with refractory DLBCL are limited. SCHOLAR-1, an international, multicohort retrospective non-Hodgkin lymphoma research study, retrospectively evaluated outcomes in patients with refractory DLBCL which, for this study, was defined as progressive disease or stable disease as best response at any point during chemotherapy (>4 cycles of first-line or 2 cycles of later-line therapy) or relapsed at ≤12 months from autologous stem cell transplantation. SCHOLAR-1 pooled data from 2 phase 3 clinical trials (Lymphoma Academic Research Organization-CORAL and Canadian Cancer Trials Group LY.12) and 2 observational cohorts (MD Anderson Cancer Center and University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence). Response rates and overall survival were estimated from the time of initiation of salvage therapy for refractory disease. Among 861 patients, 636 were included on the basis of refractory disease inclusion criteria. For patients with refractory DLBCL, the objective response rate was 26% (complete response rate, 7%) to the next line of therapy, and the median overall survival was 6.3 months. Twenty percent of patients were alive at 2 years. Outcomes were consistently poor across patient subgroups and study cohorts. SCHOLAR-1 is the largest patient-level pooled retrospective analysis to characterize response rates and survival for a population of patients with refractory DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), with 27 650 estimated new cases diagnosed in the United States in 2016 and an annual incidence of 3 to 4 per 100 000 persons in Europe.1,2 Survival rates have improved over the last several decades, with the most recent 5-year relative survival rate reported as 62.0% in the United States and 55.4% in Europe.3,4 In the immunochemotherapy era, more than 50% of patients with advanced-stage de novo DLBCL are cured with rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).5 However, depending on the number of adverse prognostic factors from the International Prognostic Score (IPI), 20% to 50% of patients with DLBCL will be refractory to R-CHOP or will relapse after achieving complete response (CR).6,7 Among patients who progress during initial immunochemotherapy or soon after a brief CR, only 30% to 40% will respond to salvage chemotherapy and may subsequently undergo consolidation with autologous stem cell transplantation (ASCT).8-10 Even among patients with relapsed or refractory DLBCL who respond to salvage therapy and are able to undergo ASCT, about 50% will ultimately relapse after transplantation.11,12 The prognosis for these patients is poor, especially for those who have high-risk factors such as secondary IPI score >2 or relapse ≤12 months post-ASCT.10,13 Thus, most patients with refractory DLBCL have no curative treatment options.14
Clinical observations suggest that patients with refractory DLBCL, defined as no response to last chemotherapy or relapse ≤12 months post-ASCT, have poor overall survival (OS) rates; however, there is a paucity of published data reporting outcomes in this patient population.13 Previous studies of patients with refractory DLBCL included small cohorts of patients.13,15-17 Despite the clinical awareness of poor outcomes in patients with refractory disease, there has never been a large-scale effort to specifically characterize response to therapy and survival outcomes in these patients. Results from clinical trials and retrospective cohort analyses identified by using a similar definition of refractory showed that these patients have consistently poor clinical outcomes.13,15-23 In 6 studies that assessed different chemotherapy regimens for 251 patients who had aggressive lymphoma refractory to first-line therapy, the objective response rate ranged from 0% to 23%, and the median OS was <10 months.15,17-20,22 In an additional 3 studies of 135 patients whose lymphoma was refractory to second-line therapy, the objective response rate ranged from 1% to 14%, and the median OS was 5 months.18,21,24 Finally, in 2 studies of patients who relapsed after ASCT, median OS of 8 months (n = 45) and 10 months (n = 75) were reported.13,23
There is an urgent need for effective treatments for patients with refractory DLBCL whose disease fails to respond to immunochemotherapy or any subsequent salvage regimen and for those whose disease relapses early post-ASCT. A key component of any such effective treatment would be a high response rate, either durable unto itself or potentially offering the opportunity for consolidation with SCT. With many promising therapies under development for refractory DLBCL, there is a need for more precise understanding of the expected response and OS rates with currently available therapies in this patient population to establish a benchmark for future studies. We constructed an international, multicohort retrospective non-Hodgkin lymphoma research study (SCHOLAR-1), which represents the largest patient-level pooled analysis to evaluate responses and OS rates in patients with refractory NHL, including DLBCL-transformed follicular lymphoma (TFL) and primary mediastinal B-cell lymphoma (PMBCL).
Methods
Study design
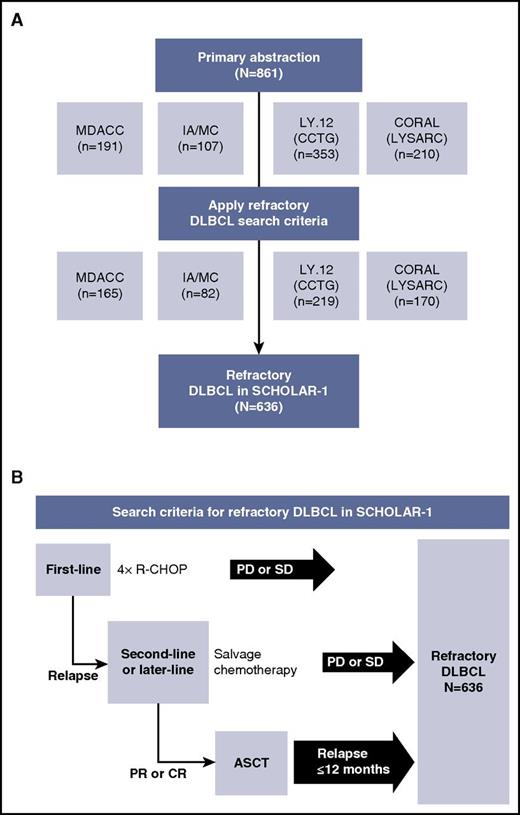
For SCHOLAR-1, patient-level data were collected for patients with refractory DLBCL from 4 sources: observational cohorts from MD Anderson Cancer Center (MDACC)25 and the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (IA/MC)26,27 and follow-up of 2 large phase 3 randomized controlled trials, Canadian Cancer Trials Group study LY.12,8 and the Lymphoma Academic Research Organization (LYSARC) Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study.9,12 Cohort details have been previously described.8,9,25,27,28 Briefly, the MDACC observational cohort included patients with DLBCL and TFL who were relapsed or refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MDACC.25 The IA/MC is a Midwest US observational cohort that enrolled unselected, newly diagnosed patients with lymphoma who then entered prospective documentation of primary and subsequent treatments and outcomes.26,27 In the international randomized LY.12 study, 619 patients (from 4 countries) were enrolled at the time of relapse after anthracycline-containing therapy and were randomly assigned to 1 of 2 salvage regimens with a goal of consolidative ASCT. The CORAL study enrolled 477 patients (from 11 countries) with DLBCL who were in their first relapse or whose lymphoma was refractory to first-line therapy, and patients were randomly assigned to 1 of 2 salvage regimens with a goal of consolidative ASCT. In the latter 2 studies, eligible patients with CD20+ lymphoma were randomly assigned to rituximab maintenance or observation after ASCT (supplemental Table 1, available on the Blood Web site). The primary abstraction method included patients with relapsed or refractory disease who were selected from the 4 study cohorts (N = 861). The authors at each institution abstracted the data according to guidelines given in the research project proposal. Upon receipt of data from individual institutions, Kite Pharma personnel programmatically applied the specific refractory search criteria to derive the SCHOLAR-1 analysis set (N = 636; Figure 1). Institutional authors were consulted for clarification of ambiguous patient cases during this process. Patient cases that were excluded were fed back to the participating authors. Abstraction criteria were programmatic confirmation of refractory status defined as progressive disease as best response to any line of chemotherapy, stable disease as best response to ≥4 cycles of first-line therapy, or 2 cycles of later-line therapy or relapse ≤12 months (365 days) after ASCT; initiation of a line of therapy after refractory status was determined; and evidence (response outcome and/or date or response) of disease assessment after initiation of therapy for refractory disease.
Patient inclusion and search criteria in SCHOLAR-1. Diagram of (A) patients with refractory DLBCL included in the SCHOLAR-1 analysis and (B) search criteria for refractory DLBCL included in SCHOLAR-1. CCTG, Canadian Cancer Trials Group; PD, progressive disease; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; SD, stable disease.
Patient inclusion and search criteria in SCHOLAR-1. Diagram of (A) patients with refractory DLBCL included in the SCHOLAR-1 analysis and (B) search criteria for refractory DLBCL included in SCHOLAR-1. CCTG, Canadian Cancer Trials Group; PD, progressive disease; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; SD, stable disease.
Patient selection
All patients from each data source who met criteria for refractory DLBCL, including TFL and PMBCL, who received subsequent therapy were considered for analysis. Refractory DLBCL (including subtypes PMBCL and TFL) was defined as progressive disease (received ≥4 cycles of first-line therapy) or stable disease (received 2 cycles of later-line therapy) as best response to chemotherapy or relapse ≤12 months after ASCT. TFL and PMBCL were included because they are histologically similar and are clinically treated as large-cell lymphoma.29-31 Patients must have received an anti-CD20 monoclonal antibody and an anthracycline as 1 of their qualifying regimens. For IA/MC, LY.12, and CORAL, patients were included at first instance of meeting refractory criteria, whereas for MDACC, patients who first met refractory criteria from second-line therapy onward were included. Patients with primary central nervous system lymphoma were excluded.
Assessments
Response to therapy for refractory disease was determined by the 1999 International Working Group response criteria per local review for randomized studies.8,9,32 Response to therapy for the observational cohorts was determined by investigator assessment also using International Working Group response criteria. In the clinical trials, patients determined to be refractory were assessed for survival approximately every 3 months for 1 year and then every 6 months for 3 years in CORAL and at least annually for LY.12 per protocol. For the observational studies, patients were followed up for disease response and survival per institution standard procedures. Patients who were alive at the time of data extraction were censored at the date of last contact.
Covariates
Covariates included IPI risk category (low risk, 0-1 point; low-intermediate risk, 2 points; high-intermediate to high risk, ≥3 points), Eastern Cooperative Oncology Group performance status (ECOG PS), stage of disease, and line of therapy before refractory status. For the observational cohorts, covariates were determined at diagnosis. For the randomized study cohorts, covariates were determined at random assignment. For all cohorts, in some cases, covariates were also measured later in the treatment course, depending on data availability and accessibility or study design. The determination of refractory status may have been distant in time from the measurement of the covariate. For summaries of patient characteristics, the covariate measured closest in time to the determination of refractory status was used. For subgroup analyses, patients were included in the covariate subgroup analysis only if the covariate was measured within 3 months of the determination of refractory status. Refractory subgroups were defined as refractory to first-line therapy, refractory to second-line or later-line therapy, or relapsed ≤12 months after ASCT. Categorization of refractory subgroup was defined by the first time a patient met the criteria for refractory disease in the IA/MC, LY.12, and CORAL study cohorts. Patients in the MDACC study cohort were categorized as either refractory to second-line or later-line therapy or relapsed ≤12 months after ASCT. The line of therapy before refractory status was determined according to the first time a patient was determined to be refractory.
Pooled analysis methodology
Patient-level data extracted by using the above criteria were submitted to a central database from which a pooled analysis was performed. For the randomized studies, responses were prospectively evaluated per the study schedule of assessments. For the observational cohorts, responses were determined at the time of patient treatment or management. Responses were obtained from an electronic medical record or patient medical record. Higgin’s Q statistic was used to assess the heterogeneity of response rate between the source databases.33 This statistic describes the percentage of variability in the effect estimates that is a result of heterogeneity rather than sampling error. A nonsignificant P value suggests that the heterogeneity does not have a strong influence on the variability in the analysis and that the data may be combined for analysis without further adjustment. In this analysis, a Higgin’s Q statistic prespecified value of P > .1 was used to determine whether significant heterogeneity was present; the P value was > .1, and thus the data were pooled for analysis. Data were pooled at the patient-record level, and response rates were estimated from the pooled data with a random effects model.34 Covariates for response were evaluated with a Cochran-Mantel-Haenszel test stratified by institution. Survival was estimated, and covariates were assessed by a Cox proportional hazards model stratified by data source. When covariates assessed after the start of therapy for refractory status were used in survival models, survival time was calculated from the day of covariate assessment. A nominal P value of .05 from the Cochran-Mantel-Haenszel tests and Cox models was used to evaluate the effect of covariates on response and survival.
Results
Demographic and baseline characteristics
Among 861 patients whose records were abstracted (MDACC, n = 191; IA/MC, n = 107; LY.12, n = 353; CORAL, n = 210), 636 were included for this analysis on the basis of refractory inclusion criteria (Figure 1). Most patients had ECOG PS ≤1 and stage III-IV disease (Table 1). Approximately one-fourth of patients had high-intermediate or high-risk IPI risk classification. There was variability among data sets; most patients (90%) from the MDACC study were refractory to second-line or later-line therapy compared with approximately half the patients in the IA/MC and CORAL studies. Less than one-third of patients in LY.12 were refractory to second-line or later-line therapy. Notably, the response rates in the DLBCL refractory groups from this analysis were lower than those from the primary analyses of LY.12 (26% for DLBCL refractory vs 45.1% for the gemcitabine, dexamethasone, and cisplatin treatment group and 44.1% for the dexamethasone, cytarabine, and cisplatin treatment group) and CORAL (31% for DLBCL refractory vs 63.5% for the rituximab, ifosfamide, etoposide, and carboplatin treatment group and 62.8% for the rituximab, dexamethasone, cytarabine, and cisplatin treatment group).8,9
Baseline patient characteristics
| Characteristic . | MDACC (n = 165) . | IA/MC (n = 82) . | LY.12 (CCTG) (n = 219) . | CORAL (LYSARC) (n = 170) . | Pooled (N = 636) . |
|---|---|---|---|---|---|
| Median age, y (range) | 56 (20-81) | 60 (20-80) | 54 (24-70) | 54 (19-65) | 55 (19-81) |
| Male sex, % | 64 | 62 | 61 | 69 | 64 |
| Primary diagnosis, % | |||||
| DLBCL* | 76 | 89 | 84 | 100 | 87 |
| PMBCL | 1 | 0 | 5 | 0 | 2 |
| TFL | 3 | 0 | 10 | 0 | 4 |
| Indeterminate/missing | 19 | 11 | 1 | 0 | 7 |
| ECOG PS, % | |||||
| 0-1 | 42 | 72 | 89 | 84 | 73 |
| 2-4 | 10 | 24 | 11 | 15 | 14 |
| Missing | 49 | 4 | 0 | 1 | 13 |
| Disease stage, % | |||||
| I-II | 18 | 20 | 33 | 32 | 27 |
| III-IV | 82 | 79 | 67 | 67 | 72 |
| Missing | 0 | 1 | 0 | 1 | <1 |
| IPI risk classification, %† | |||||
| Low risk | 5 | 22 | 36 | 32 | 25 |
| Low-intermediate risk | 7 | 31 | 30 | 29 | 24 |
| High-intermediate to high risk | 23 | 48 | 35 | 34 | 33 |
| Missing or incompletely assessed | 65 | 0 | 0 | 5 | 18 |
| Refractory category, % | |||||
| Primary refractory | 0 | 24 | 51 | 28 | 28 |
| Refractory to ≥ second-line therapy | 90 | 51 | 21 | 46 | 50 |
| Relapsed ≤12 mo post-ASCT | 10 | 24 | 28 | 26 | 22 |
| Total no. of lines of chemotherapy and ASCT received, %‡ | |||||
| 1 | 0 | 24 | 51 | 28 | 28 |
| 2-3 | 90 | 50 | 21 | 46 | 49 |
| ≥4 | 0 | 1 | 0 | 0 | <1 |
| Characteristic . | MDACC (n = 165) . | IA/MC (n = 82) . | LY.12 (CCTG) (n = 219) . | CORAL (LYSARC) (n = 170) . | Pooled (N = 636) . |
|---|---|---|---|---|---|
| Median age, y (range) | 56 (20-81) | 60 (20-80) | 54 (24-70) | 54 (19-65) | 55 (19-81) |
| Male sex, % | 64 | 62 | 61 | 69 | 64 |
| Primary diagnosis, % | |||||
| DLBCL* | 76 | 89 | 84 | 100 | 87 |
| PMBCL | 1 | 0 | 5 | 0 | 2 |
| TFL | 3 | 0 | 10 | 0 | 4 |
| Indeterminate/missing | 19 | 11 | 1 | 0 | 7 |
| ECOG PS, % | |||||
| 0-1 | 42 | 72 | 89 | 84 | 73 |
| 2-4 | 10 | 24 | 11 | 15 | 14 |
| Missing | 49 | 4 | 0 | 1 | 13 |
| Disease stage, % | |||||
| I-II | 18 | 20 | 33 | 32 | 27 |
| III-IV | 82 | 79 | 67 | 67 | 72 |
| Missing | 0 | 1 | 0 | 1 | <1 |
| IPI risk classification, %† | |||||
| Low risk | 5 | 22 | 36 | 32 | 25 |
| Low-intermediate risk | 7 | 31 | 30 | 29 | 24 |
| High-intermediate to high risk | 23 | 48 | 35 | 34 | 33 |
| Missing or incompletely assessed | 65 | 0 | 0 | 5 | 18 |
| Refractory category, % | |||||
| Primary refractory | 0 | 24 | 51 | 28 | 28 |
| Refractory to ≥ second-line therapy | 90 | 51 | 21 | 46 | 50 |
| Relapsed ≤12 mo post-ASCT | 10 | 24 | 28 | 26 | 22 |
| Total no. of lines of chemotherapy and ASCT received, %‡ | |||||
| 1 | 0 | 24 | 51 | 28 | 28 |
| 2-3 | 90 | 50 | 21 | 46 | 49 |
| ≥4 | 0 | 1 | 0 | 0 | <1 |
CCTG, Canadian Cancer Trials Group; LYSARC, Lymphoma Academic Research Organization.
In the CORAL (LYSARC) study, the disease subtype for 96 patients was not available; per the study inclusion criteria, patients were to have DLBCL.
IPI was determined at diagnosis for MDACC and IA/MC and at randomization for LY.12 and CORAL study patients; low risk, 0-1 point; low-intermediate risk, 2 points; high-intermediate to high risk, ≥3 points.
Includes the 78% of patients who were refractory to chemotherapy and excludes those who relapsed post-ASCT.
Response rates
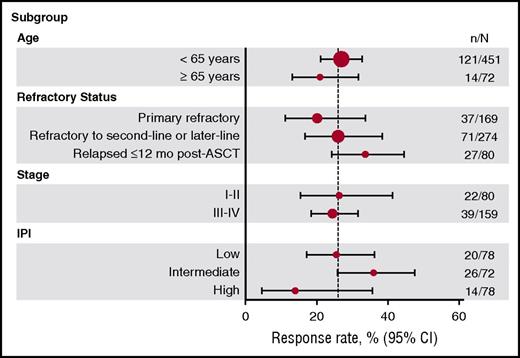
Response rates were similar across the 4 data sets, ranging from 20% to 31%, with a pooled response rate of 26% (Table 2). CR rates ranged from 2% to 15%, with a pooled CR rate of 7%. Pooled response rates by refractory subgroup (primary refractory, refractory to second-line or later-line therapy, and relapsed ≤12 months after ASCT) ranged from 20% to 39%. Response rates were consistently low across all subgroups, with the lowest response rates in the primary refractory and high-risk IPI subgroups (Figure 2). Patients who relapsed ≤12 months after ASCT had higher response rates (34%) than those in the primary refractory (20%) or second-line therapy or greater (26%) groups (Table 2).
Rate of response to chemotherapy after refractory disease
| . | MDACC (n = 165) . | IA/MC (n = 82) . | LY.12 (CCTG) (n = 219) . | CORAL (LYSARC) (n = 170) . | Pooled* (N = 636) . |
|---|---|---|---|---|---|
| Patients evaluated for response, n† | 165 | 82 | 106 | 170 | 523 |
| Response rate, % (95% CI) | 20 | 26 | 26 | 31 | 26 (21-31) |
| CR rate | 7 | 7 | 2 | 15 | 7 (3-15) |
| PR rate | 13 | 18 | 25 | 16 | 18 (13-23) |
| Response rate by refractory category, % (95% CI) | |||||
| Primary refractory | |||||
| RR | — | 25 | 27 | 10 | 20 (11-34) |
| CR rate | — | 10 | 1 | 2 | 3 (1-11) |
| Refractory to second-line or later-line therapy | |||||
| RR | 20 | 21 | 20 | 40 | 26 (17-39) |
| CR rate | 7 | 5 | 20 | 18 | 10 (5-20) |
| Relapse ≤12 mo post-ASCT | |||||
| RR | 19 | 35 | — | 39 | 34 (24-45) |
| CR rate | 6 | 10 | — | 25 | 15 (6-31) |
| . | MDACC (n = 165) . | IA/MC (n = 82) . | LY.12 (CCTG) (n = 219) . | CORAL (LYSARC) (n = 170) . | Pooled* (N = 636) . |
|---|---|---|---|---|---|
| Patients evaluated for response, n† | 165 | 82 | 106 | 170 | 523 |
| Response rate, % (95% CI) | 20 | 26 | 26 | 31 | 26 (21-31) |
| CR rate | 7 | 7 | 2 | 15 | 7 (3-15) |
| PR rate | 13 | 18 | 25 | 16 | 18 (13-23) |
| Response rate by refractory category, % (95% CI) | |||||
| Primary refractory | |||||
| RR | — | 25 | 27 | 10 | 20 (11-34) |
| CR rate | — | 10 | 1 | 2 | 3 (1-11) |
| Refractory to second-line or later-line therapy | |||||
| RR | 20 | 21 | 20 | 40 | 26 (17-39) |
| CR rate | 7 | 5 | 20 | 18 | 10 (5-20) |
| Relapse ≤12 mo post-ASCT | |||||
| RR | 19 | 35 | — | 39 | 34 (24-45) |
| CR rate | 6 | 10 | — | 25 | 15 (6-31) |
Response rate to the line of therapy was given after determination of refractory status.
CI, confidence interval; RR, response rate.
Higgin’s Q statistic, P = .18.
Data contain evidence that the patient proceeded to salvage therapy, but response information was not collected.
Response rates by subgroup. The vertical line within the plot represents the objective response rate of 26%. CI, confidence interval; N, the total number of patients in the patient subgroup; n, the number of patients with response.
Response rates by subgroup. The vertical line within the plot represents the objective response rate of 26%. CI, confidence interval; N, the total number of patients in the patient subgroup; n, the number of patients with response.
OS
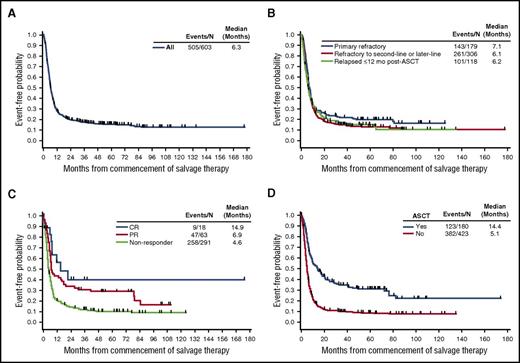
Survival from the start of salvage therapy for refractory disease was consistently poor in patients with refractory DLBCL, with a median OS of 6.3 months (95% confidence interval [CI], 5.9-7.0 months) from the start of therapy (Figure 3A). The 1-year survival rate was 28%, and 20% remained alive at 2 years (Table 3). OS rates were similar regardless of refractory subgroup, with a slightly lower median OS among patients who were refractory to second-line or later-line therapy or who relapsed ≤12 months after ASCT (6.1 and 6.2 months, respectively) than among primary refractory patients (7.1 months; Figure 3B). Although a higher response rate among patients in the post-ASCT group was observed, the survival in the post-ASCT group was similar to that of the other refractory subgroups evaluated.
Overall survival from commencement of salvage therapy. Shown for the (A) overall population, (B) refractory subgroups, (C) tumor response, and (D) post-refractory transplantation status (Kaplan-Meier).
Overall survival from commencement of salvage therapy. Shown for the (A) overall population, (B) refractory subgroups, (C) tumor response, and (D) post-refractory transplantation status (Kaplan-Meier).
Overall survival by relapsed/refractory status
| . | MDACC (n = 165) . | IA/MC (n = 82) . | LY.12 (CCTG) (n = 219) . | CORAL (LYSARC) (n = 170) . | Pooled (N = 636) . |
|---|---|---|---|---|---|
| Patients evaluated for survival, n | 165 | 72 | 196 | 170 | 603 |
| Survival from start of salvage therapy | |||||
| Deaths | 89 | 92 | 80 | 80 | 84 |
| Median, mo (95% CI) | 6.6 | 5.0 | 6.6 | 6.5 | 6.3 (5.9-7.0) |
| 1-y survival rate | 28 | 18 | 31 | 30 | 28 (25-32) |
| 2-y survival rate | 17 | 10 | 23 | 22 | 20 (16-23) |
| Primary refractory | |||||
| Deaths | — | 90 | 76 | 85 | 80 |
| Median, mo (95% CI) | — | 6.1 | 7.9 | 7.3 | 7.1 (6.0-8.1) |
| 1-y survival rate | — | 26 | 30 | 27 | 29 (22-36) |
| 2-y survival rate | — | 21 | 27 | 16 | 24 (18-30) |
| Refractory to second-line or greater therapy | |||||
| Deaths | 88 | 92 | 86 | 77 | 85 |
| Median, mo (95% CI) | 6.6 | 4.7 | 5.3 | 6.1 | 6.1 (5.2-7.0) |
| 1-y survival rate | 29 | 9 | 24 | 30 | 26 (22-31) |
| 2-y survival rate | 19 | 6 | 14 | 22 | 17 (13-22) |
| Relapse at 12 months post-ASCT or earlier | |||||
| Deaths | 94 | 94 | 86 | 80 | 86 |
| Median, mo (95% CI) | 5.9 | 4.2 | 7.0 | 6.5 | 6.2 (5.2-7.6) |
| 1-y survival rate | 19 | 25 | 38 | 34 | 32 (24-41) |
| 2-y survival rate | 6 | 6 | 21 | 26 | 19 (12-27) |
| . | MDACC (n = 165) . | IA/MC (n = 82) . | LY.12 (CCTG) (n = 219) . | CORAL (LYSARC) (n = 170) . | Pooled (N = 636) . |
|---|---|---|---|---|---|
| Patients evaluated for survival, n | 165 | 72 | 196 | 170 | 603 |
| Survival from start of salvage therapy | |||||
| Deaths | 89 | 92 | 80 | 80 | 84 |
| Median, mo (95% CI) | 6.6 | 5.0 | 6.6 | 6.5 | 6.3 (5.9-7.0) |
| 1-y survival rate | 28 | 18 | 31 | 30 | 28 (25-32) |
| 2-y survival rate | 17 | 10 | 23 | 22 | 20 (16-23) |
| Primary refractory | |||||
| Deaths | — | 90 | 76 | 85 | 80 |
| Median, mo (95% CI) | — | 6.1 | 7.9 | 7.3 | 7.1 (6.0-8.1) |
| 1-y survival rate | — | 26 | 30 | 27 | 29 (22-36) |
| 2-y survival rate | — | 21 | 27 | 16 | 24 (18-30) |
| Refractory to second-line or greater therapy | |||||
| Deaths | 88 | 92 | 86 | 77 | 85 |
| Median, mo (95% CI) | 6.6 | 4.7 | 5.3 | 6.1 | 6.1 (5.2-7.0) |
| 1-y survival rate | 29 | 9 | 24 | 30 | 26 (22-31) |
| 2-y survival rate | 19 | 6 | 14 | 22 | 17 (13-22) |
| Relapse at 12 months post-ASCT or earlier | |||||
| Deaths | 94 | 94 | 86 | 80 | 86 |
| Median, mo (95% CI) | 5.9 | 4.2 | 7.0 | 6.5 | 6.2 (5.2-7.6) |
| 1-y survival rate | 19 | 25 | 38 | 34 | 32 (24-41) |
| 2-y survival rate | 6 | 6 | 21 | 26 | 19 (12-27) |
Values shown as percentages, unless otherwise indicated. Hazard ratios (HRs) have been calculated from a Cox proportional hazards model (stratified by center). Second-line refractory vs primary refractory, HR, 1.24 (P = .17). Relapsed ≤12 months after ASCT vs primary refractory, HR, 1.20 (P = .17).
To better characterize factors driving the long-term survival rates, we evaluated OS within different patient subgroups (tumor response, post-refractory transplantation status, age, ECOG PS, and IPI risk category). Although they represent a relatively small proportion of patients, those who achieved a CR after last salvage chemotherapy had longer survival (median OS, 14.9 months) compared with nonresponders (median OS, 4.6 months; Figure 3C). The 2-year OS rate for nonresponders was 14% (Figure 3C). Median OS was higher among the 180 patients who had undergone ASCT (14.4 months) than among the 423 patients who had not undergone ASCT; the median OS of the latter group was 5.1 months (Figure 3D), and the 2-year OS rate was 11% (95% CI, 8%-14%; Figure 3D). Thirty-one patients who achieved a CR underwent ASCT, and their median OS was more than 6 years at the time of this analysis. Of the 54 patients who achieved a partial response (PR) and underwent ASCT, the median OS was 17.8 months (supplemental Figure 1). For the 89 patients (18%) who were unable to achieve a CR or PR and who underwent ASCT after receiving an intervening line of therapy, the median OS was 8.7 months. Fifty-seven patients who received ASCT were alive at last follow-up (range, 1-14 years). Other factors that were significantly different for OS included ECOG PS (0-1 vs ≥2, P < .0001), disease stage (I-II vs III-IV, P < .0001), and IPI risk groups (low vs low-intermediate, P < .0001; low-intermediate vs high-intermediate, P < .01) (Table 4; supplemental Figure 2). Age younger than 65 years or age 65 years or older did not impact OS.
Overall survival by patient subgroup
| . | Pooled (N = 636) . | |||
|---|---|---|---|---|
| . | Median OS, mo (95% CI) . | 1-y OS rate, % (95% CI) . | 2-y OS rate, % (95% CI) . | Hazard ratio* (P) . |
| Age | ||||
| <65 y | 6.3 (5.8-7.0) | 28 (24-32) | 20 (16-23) | 1 (reference) |
| ≥65 y | 6.9 (4.9-9.5) | 30 (20-40) | 19 (11-29) | 0.9 (.37) |
| ECOG PS | ||||
| 0-1 | 6.5 (6.0-7.6) | 27 (22-33) | 21 (16-26) | 1 (reference) |
| 2+ | 3.0 (2.0-4.3) | 15 (7-24) | 6 (2-14) | 2.1 (<.0001) |
| Disease stage | ||||
| I-II | 8.7 (6.5-11.3) | 39 (29-49) | 31 (22-40) | 1 (reference) |
| III-IV | 5.4 (4.3-6.2) | 20 (15-26) | 15 (10-20) | 1.8 (<.0001) |
| IPI risk classification† | ||||
| Low risk | 9.6 (7.4-16.6) | 44 (34-54) | 35 (26-45) | 1 (reference) |
| Low-intermediate risk | 6.3 (5.2-8.2) | 22 (13-31) | 15 (8-24) | 1.8 (<.0001) |
| High-intermediate to high risk | 3.8 (2.9-5.0) | 15 (9-23) | 10 (5-18) | 2.8 (<.01) |
| . | Pooled (N = 636) . | |||
|---|---|---|---|---|
| . | Median OS, mo (95% CI) . | 1-y OS rate, % (95% CI) . | 2-y OS rate, % (95% CI) . | Hazard ratio* (P) . |
| Age | ||||
| <65 y | 6.3 (5.8-7.0) | 28 (24-32) | 20 (16-23) | 1 (reference) |
| ≥65 y | 6.9 (4.9-9.5) | 30 (20-40) | 19 (11-29) | 0.9 (.37) |
| ECOG PS | ||||
| 0-1 | 6.5 (6.0-7.6) | 27 (22-33) | 21 (16-26) | 1 (reference) |
| 2+ | 3.0 (2.0-4.3) | 15 (7-24) | 6 (2-14) | 2.1 (<.0001) |
| Disease stage | ||||
| I-II | 8.7 (6.5-11.3) | 39 (29-49) | 31 (22-40) | 1 (reference) |
| III-IV | 5.4 (4.3-6.2) | 20 (15-26) | 15 (10-20) | 1.8 (<.0001) |
| IPI risk classification† | ||||
| Low risk | 9.6 (7.4-16.6) | 44 (34-54) | 35 (26-45) | 1 (reference) |
| Low-intermediate risk | 6.3 (5.2-8.2) | 22 (13-31) | 15 (8-24) | 1.8 (<.0001) |
| High-intermediate to high risk | 3.8 (2.9-5.0) | 15 (9-23) | 10 (5-18) | 2.8 (<.01) |
Hazard ratio from a Cox proportional hazards model stratified by center.
Low risk, 0-1 points; low-intermediate risk, 2 points; high-intermediate to high risk, ≥3 points.
Discussion
Although individual clinical observations suggest that survival rates are poor among patients with refractory DLBCL, published comprehensive data are limited for this patient population. Previous studies have included only small numbers of patients, indicating a need for a larger analysis of outcomes in patients with refractory DLBCL. The results of studies of patients with DLBCL refractory to second-line therapy or who relapsed after ASCT have shown poor clinical outcomes (median OS, 5 months and 8-10 months, respectively).10,13,16,18 The SCHOLAR-1 study is the largest, patient-level pooled analysis to evaluate response and survival rates in patients with refractory DLBCL. These data are particularly important because they represent a large number of patients treated in the modern rituximab era, suggesting that even with the availability of multiple rituximab-based regimens, outcomes among patients with refractory DLBCL remain dismal across global centers and trials.
The pooled objective response rate to the next line of therapy in our study cohort was 26% (CR, 7%), and the pooled median OS from refractory disease was 6.3 months. Outcomes were poor regardless of subgroup within our definition of refractory, with a pooled response rate of 20% (CR, 3%; PR, 17%) among primary refractory patients and 34% (CR, 15%; PR, 19%) among patients who progressed ≤12 months post-ASCT. Median OS was consistently short in the pooled population and in all patient subgroups (median OS, <10 months) across known prognostic factors and refractory status. To mitigate the potential bias, landmark survival rates were calculated from transplantation to survival outcome. Response to therapy was significantly associated with longer survival, particularly for patients who submitted to ASCT thereafter. These results showed that 20% of patients remained alive at 2 years; however, these long-term durable responses were primarily driven by the minority of patients who received ASCT and/or achieved a CR or PR and who represent the tail of the Kaplan-Meier curve of OS. Most patients (73%) did not respond to salvage therapy or were not able to receive ASCT, resulting in particularly poor outcomes. As expected, patients with higher stage disease, worse ECOG PS, and more IPI risk factors had shorter survival. There is an urgent unmet need to improve salvage regimens that may increase the percentage of patients eligible for SCT and also to develop novel and effective therapeutic options to treat this patient population.10,35
This study used a definition for refractory disease that included patients who relapsed early (within 12 months of ASCT). Smaller studies have shown that, similar to patients with DLBCL refractory to therapy in the more traditional sense, those who relapse early after receiving ASCT have poor outcomes.13,23 This study was intended to globally characterize outcomes of patients for whom the most recent therapy was minimally effective. Our results confirm our assumptions built on these earlier studies, that patients do indeed have poor outcomes, regardless of refractory subgroup.
The strength of this study is the use of individual patient data from 2 large clinical observational cohorts (IA/MC and MDACC) that provided follow-up for patients from case ascertainment to death and from 2 large, prospective, randomized phase 3 trials (LY.12 and CORAL) that evaluated salvage therapy and ASCT in DLBCL. A patient may be refractory at many times during the course of his or her disease, such as primary refractory, refractory to second-line therapy, or refractory to third-line therapy. For this analysis, a patient was considered refractory at the first possible time at which refractory criteria were met, because this maximized the information available for response and survival after determination of refractory status. A proportion of patients in this study (27%) underwent salvage therapy and ASCT for primary refractory DLBCL and remained in remission. Sensitivity analyses that excluded this cohort suggest that outcomes are worse in the remaining patients who are not successfully rescued with second-line therapy and ASCT.
Despite potential differences in patient populations and study design, outcomes were quite homogeneous. To understand the relatively favorable outcomes of some of the clinically defined subgroups in this data set, biomarkers such as cell of origin or the presence of chromosomal translocations involving BCL-2 and C-MYC (double-hit lymphomas) would be required36 ; such an evaluation of biology of patients in the SCHOLAR-1 study cohorts is planned. The incidence of C-MYC rearrangement was 17% in patients analyzed in the CORAL study population and was associated, alone or in combination with BCL-2 and or BCL-6 translocations, with a significantly inferior prognosis with standard salvage therapy.28 Results of a study of patients with DLBCL who experienced primary treatment failure showed that primary progression, intermediate-high or high IPI at the time of primary treatment failure, or MYC translocation predicted a 2-year OS rate of 13.6% and constituted ultra-high risk features.37 Although these high-risk populations were not specifically assessed in our evaluation, the poor outcomes among patients observed in this study mirror those observed in patients with high-risk factors and support the homogeneous and strikingly grave prognosis for patients with refractory DLBCL.
Although future prospective studies may be conducted in select subgroups of this patient population, this report provides extensive subgroup analyses based on patient characteristics and treatment patterns that investigators may use to provide benchmarks for future prospective studies in selected patient populations. Like all retrospective studies, limitations of the direct applicability of the results to future studies may exist. For example, the time period of patients’ treatment may influence the applicability of the benchmark to future studies. Nevertheless, the SCHOLAR-1 study provides a historical benchmark for future studies in refractory DLBCL, TFL, and PMBCL. The consistently poor outcomes shown here indicate a significant unmet need for effective therapies for patients with refractory NHL.
Presented in part at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, 3-7 June 2016; 21st Annual Meeting of the European Hematology Association, Copenhagen, Denmark, 9-12 June 2016; Pan Pacific Lymphoma Conference, Koloa, HI, 18-22 July 2016; Society of Hematologic Oncology Fourth Annual Meeting, Houston, TX, 7-10 September 2016; and Lymphoma & Myeloma 2016: An International Congress on Hematologic Malignancies, New York, NY, 13-15 October 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the study, their family, friends, and caregivers, and the study staff and health care providers.
This work was supported in part by Kite Pharma. The IA/MC Specialized Program of Research Excellence cohort was supported by National Institutes of Health, National Cancer Institute (NCI) grant P50 CA97274 and the MD Anderson Cancer Center–NCI support grant P30 CA016672. Dustin Khiem (Kite Pharma) and Jennifer Leslie and Cathy Winter (both of Nexus Global Group funded by Kite Pharma) provided medical writing support.
Authorship
Contribution: L.N., J.W., and W.Y.G. analyzed and interpreted the data; L.N. performed statistical analyses; M.C., L.N., J.W., W.Y.G., and C.G. contributed to writing the first draft of the manuscript; M.C., U.F., B.K.L., A.H., M.J.M., and C.G. provided a critical review of the manuscript’s content; and all authors reviewed and revised the manuscript and approved the final manuscript. All authors participated in designing the study including defining patient selection criteria, response definitions/determinations, follow-up methodology, and data collection. Each contributing group abstracted patient-level data by using criteria defined in the SCHOLAR research proposal and, in some cases, by using a standardized, investigator-developed data entry form; they then submitted the data to a central database from which a pooled analysis was performed by Kite Pharma. Statistical analysis review was conducted by the Canadian Cancer Trials Group.
Conflict-of-interest disclosure: S.S.N. received research funding from and served as a consultant and an advisory board member for Kite Pharma. U.F. received research funding from Kite Pharma. J.K. received research funding from Celgene, Roche, and Karyopharm; served as a consultant for Bristol-Myers Squibb, Gilead, Janssen, Hoffman LaRoche, and Seattle Genetics; and received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Gilead, Roche, Janssen, Lundbeck, Merck, and Seattle Genetics. J.W. served on advisory boards for ProNAi Therapeutics, Celgene, and Genentech and received research funding from Janssen, Celgene, Genentech, Kite, and Novartis. B.K.L., J.R.C., and M.J.M. received research funding from Kite Pharma. L.N., J.W., and W.Y.G. are employed by and have equity ownership in Kite Pharma. The remaining authors declare no competing financial interests.
Correspondence: Christian Gisselbrecht, Saint Louis Hospital, 1 Avenue Claude Vellefaux, 75010 Paris, France, and LYSARC, Lyon-Sud Hospital, Sector Sainte Eugenie, Pavillion 6D, 69495 Pierre-Benite Cedex, France; e-mail: christian.gisselbrecht@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal