Key Points
CD207+CD1a+ cells are present in the circulation of patients with active LCH.
TGF-β and TSLP levels are increased in the plasma of patients with active LCH.
Abstract
Langerhans cell histiocytosis (LCH) is a rare disease with an unknown etiology characterized by heterogeneous lesions containing CD207+CD1a+ cells that can arise in almost any tissue and cause significant morbidity and mortality. Precursors of pathological Langerhans cells have yet to be defined. Our aim was to identify circulating CD207+CD1a+ cells and their inducers in LCH. Expression of CD207 and CD1a in the blood myeloid compartment as well as thymic stromal lymphopoietin (TSLP) and transforming growth factor β (TGF-β) plasma levels were measured in 22 pediatric patients with active disease (AD) or nonactive disease (NAD). In patients with AD vs those with NAD, the myeloid compartment showed an increased CD11b (CD11bhigh plus CD11b+) fraction (39.7 ± 3.6 vs 18.6 ± 1.9), a higher percentage of circulating CD11bhighCD11c+CD207+ cells (44.5 ± 11.3 vs 3.2 ± 0.5), and the presence of CD11chighCD207+CD1a+ cells (25.0 ± 9.1 vs 2.3 ± 0.5). Blood CD207+CD1a+ cells were not observed in adult controls or umbilical cord. Increased TSLP and TGF-β levels were detected in patients with AD. Interestingly, plasma from patients with AD induces CD207 expression on CD14+ monocytes. We conclude that CD207+CD1a+ cells are circulating in patients with active LCH, and TSLP and TGF-β are potential drivers of Langerhans-like cells in vivo.
Introduction
Langerhans cell histiocytosis (LCH) remains a poorly understood disorder with heterogeneous clinical presentations that occurs predominantly in the pediatric population. LCH is characterized by invasive lesions with multiple cell type infiltrations, including pathological CD1a+CD207+ cells affecting one or several organs.1-5 The etiology of LCH is unknown, and debate centers on LCH resulting from either a malignant transformation of Langerhans cell (LC) precursors or as consequence of a deregulated immune response giving rise to pathological LCs from immature myeloid cells.2,6,7 LCs are potent antigen-presenting cells of the epidermis that self-renew in the steady state8 but are recruited in waves after inflammation from the circulation. An initial wave derives from monocytes and develops low langerin (CD207) expression,9,10 while the second wave may be derived from a CD1c+ dendritic cell (DC) precursor, which could be responsible for the long-term reconstitution of LCs.11,12
Human CD14+ monocytes can, in vitro, differentiate into CD207+ cells in response to granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and transforming growth factor β (TGF-β).13 Additionally, 2 recent articles showed that CD1c+ cells acquire an LC-like phenotype in response to either thymic stromal lymphopoietin (TSLP) and TGF-β14 or GM-CSF and bone morphogenetic protein 7,15 suggesting a potential precursor role of blood myeloid progenitors.12
When inflammation is the initiating factor, CD207+ cells and their precursors should circulate in the peripheral blood of patients with active disseminated LCH. Based on this premise, we evaluated the presence of CD207+ and CD1a+ cells in the myeloid compartment of patients with LCH, plasma levels of TGF-β and TSLP in these patients, and whether plasma could drive the differentiation of CD207+ LC-like cells.
Study design
For this study, 22 patients were recruited from the Pediatrics Department of the Hospital de Clínicas “Jose de San Martin” and Hospital de Niños “Pedro de Elizalde,” Buenos Aires, Argentina. All patients met Histiocyte Society LCH III Protocol criteria for diagnosis, stratification and disease progression status.16,17 Median age was 2.07 years (range, 0.25-11.62 years), and 13 patients were female. The disease state of patients, defined as active disease (AD) or nonactive disease (NAD), and treatments are outlined in supplemental Table 1 (available on the Blood Web site). C-reactive protein determination and erythrocyte sedimentation rate are included in supplemental Table 1, and comparative analysis between patients with NAD and AD was performed. The number of platelets was also compared (supplemental Figure 1A-C). Informed consent was obtained from parents or legal guardians of the patients. This project was approved by the corresponding local ethics committees and institutional review boards. Additionally, peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers under approval of local ethics committees.
PBMCs were isolated from 1 to 3 mL anticoagulated blood by Ficoll gradient centrifugation. Plasma from each sample was collected and stored at −80°C for cytokine analysis. Flow cytometry was performed using FACS Canto (Becton Dickinson), and antibodies were purchased from BioLegend. Myeloid and T-cell compartments were analyzed using the appropriate combination of CD11b phycoerythrin (PE)/Cy7 (M1/70), CD11b allophycocyanin (APC)/Cy7 (M1/70), CD11c-PercP (3.9), CD14-PE/Cy7 (HCD14), CD2-PercP (RPA-2.10), CD4-APC/Cy7 (OKT4), CD1c-PE/Cy7 (L161), CD207-APC (10E2), and CD1a-PE (HI149).
TGF-β and TSLP plasma levels were determined using ELISA Ready-SET-Go! (eBioscience).
CD207 and CD1a induction was assessed on sorted CD14+ monocytes (EasySep Human CD14 Positive Selection Kit) or total PBMCs by plating 1 × 105 CD14+ cells or 1 × 106 PBMCs in 500 µL RPMI supplemented with 10% fetal bovine serum and 1% antibiotics. Plasma from patients with AD or NAD was added at 10% vol/vol from the beginning of the culture. Cells were harvested at days 4, 7, and 10.
Results and discussion
Increased circulating CD1a and CD207 myeloid cells in patients with active LCH
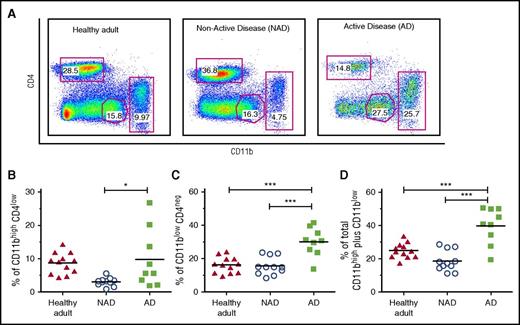
In order to characterize and compare the myeloid and T-cell compartments of patients with active and nonactive LCH, PBMCs were stained with CD11b, CD2, and CD4 lineage markers. The analysis showed a significant increase in the CD11b fraction, including cells expressing CD11bhigh and CD11bint/low, in patients with AD compared with patients with NAD (39.7 ± 3.6 vs 18.6 ± 1.9, P < .001) and healthy adults (24.9 ± 1.3, P < .001) (Figure 1A-D). The absolute number of blood CD11b cells was also significantly increased (supplemental Figure 2A). Another myeloid population, CD11blowCD2int, which was previously described as a subtype of circulating DCs,18 was also significantly increased in patients with AD (supplemental Figure 2B-C). These data indicate an active myelopoiesis process in patients with AD concomitant with a decrease in the T-cell compartment (data not shown).
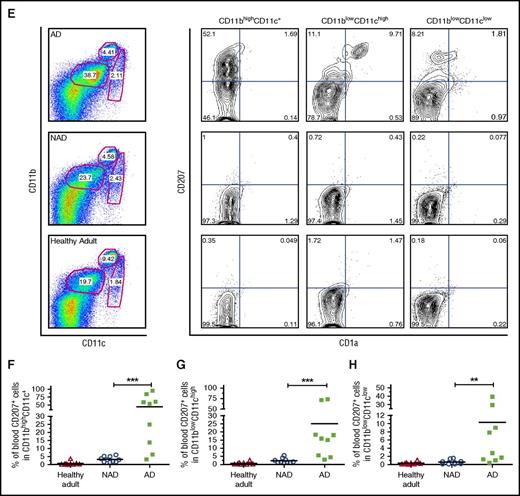
Increased CD11b fraction and circulating CD207+CD1a+myeloid cells in peripheral blood of patients with active LCH. (A) Gating strategy used to discriminate CD11b myeloid lineage and the CD4 T-cell compartment. (B-D) Independent data showing percentage of CD11bhighCD4low; CD11blowCD4neg, and CD11bhigh plus CD11blow cells in patients with AD (N = 9) compared with patients with NAD (N = 11) and healthy adults (N = 12). Unpaired 2-tailed t test was performed to compare AD patients with NAD patients or healthy adults. *P < .05; ***P < .001. (E) Representative dot plot and gating strategies used to discriminate CD11b and CD11c subpopulations from PBMCs. The expression of CD207 and CD1a was analyzed in CD11bhighCD11c+ monocytes, CD11blowCD11chigh DCs, and CD11blowCD11clow cells. (F-H) Independent data for positive CD207 cells, including CD207+ plus CD207+CD1a+ (Q1+Q2), for each gated subpopulation in AD patients (N = 9), NAD patients (N = 10), and healthy adults (N = 9) are shown. A 2-tailed Mann-Whitney test was performed (**P < .01; ***P < .001).
Increased CD11b fraction and circulating CD207+CD1a+myeloid cells in peripheral blood of patients with active LCH. (A) Gating strategy used to discriminate CD11b myeloid lineage and the CD4 T-cell compartment. (B-D) Independent data showing percentage of CD11bhighCD4low; CD11blowCD4neg, and CD11bhigh plus CD11blow cells in patients with AD (N = 9) compared with patients with NAD (N = 11) and healthy adults (N = 12). Unpaired 2-tailed t test was performed to compare AD patients with NAD patients or healthy adults. *P < .05; ***P < .001. (E) Representative dot plot and gating strategies used to discriminate CD11b and CD11c subpopulations from PBMCs. The expression of CD207 and CD1a was analyzed in CD11bhighCD11c+ monocytes, CD11blowCD11chigh DCs, and CD11blowCD11clow cells. (F-H) Independent data for positive CD207 cells, including CD207+ plus CD207+CD1a+ (Q1+Q2), for each gated subpopulation in AD patients (N = 9), NAD patients (N = 10), and healthy adults (N = 9) are shown. A 2-tailed Mann-Whitney test was performed (**P < .01; ***P < .001).
Surprisingly, we detected circulating CD207+ myeloid cells in the blood of patients with AD. Significantly higher percentages of circulating CD11bhighCD11c+CD207+ monocytes and CD11blowCD11chighCD207+CD1a+ DCs were present in patients with AD than in those with NAD, (44.6 ± 11.3 vs 3.2 ± 0.5 and 25.0 ± 9.1 vs 2.3 ± 0.5, respectively; P < .001). Representative dot plots, gating strategy, and independent data are shown in Figure 1E-H. Additionally, mononuclear cells from umbilical cord blood did not show expression of CD207 or CD1a in any of the subpopulations assessed (supplemental Figure 3). Remarkably, we were unable to detect CD207- and CD1a-positive cells in PBMCs of 3 patients with disseminated juvenile xanthogranuloma, a non-LCH disease (data not shown). In summary our data indicate that CD207+CD1a+ myeloid cells circulate in patients with active LCH, suggesting a potential specific marker for active disseminated disease.
Higher levels of plasma TGF-β and TSLP were detected in patients with active LCH and correlated with the percentage of CD207-CD1a–expressing cells
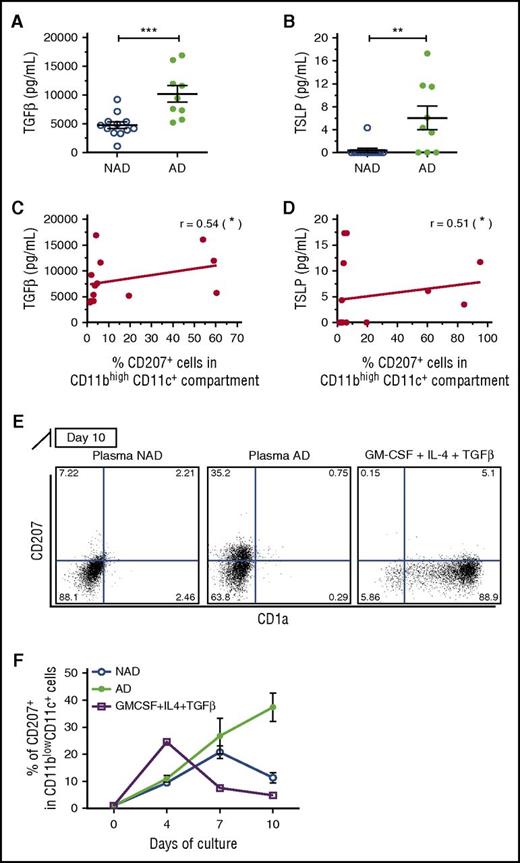
Considering that TGF-β and TSLP were recently described as 2 preponderant cytokines for in-vitro LC differentiation,14 we measured both cytokines in plasma of patients with AD and NAD LCH. We found higher levels of TGF-β and TSLP (Figure 2A-B) in patients with AD. Moreover, the levels of both cytokines correlated positively with the percentage of positive CD207 plus CD1a–expressing monocytes (CD11bhighCD11c+) (Figure 2C-D). These data suggest that TGF-β and TSLP could be driving the induction of CD207 and CD1a in circulating myeloid cells during the pathogenesis of LCH.
Increased plasma levels of TGF-β and TSLP correlate with the increased percentage of CD207 expressing cells in patients with active LCH. (A-B) Plasma levels of TGF-β and TSLP were measured by enzyme-linked immunosorbent assay, and independent data from patients with NAD (N = 12) and AD (N = 9) are graphed. (C-D) Plasma levels of TGF-β and TSLP correlated with the percentage of monocytes (CD11bhighCD11c+) expressing CD207 (CD207+ plus CD207+CD1a+) (Spearman test). (E-F) CD14+ monocytes were isolated from healthy volunteers and cultured in the presence of plasma from patients with AD or NAD (10% vol/vol) for 4 to 10 days. CD207 and CD1a expression was evaluated in CD11c+CD11blow cells by flow cytometry. (E) Representative dot plot of CD207 vs CD1a at day 10. GM-CSF, IL-4, and TGF-β were used as positive controls (see also supplemental Figure 3A). (F) Kinetic expression of CD207 at days 4, 7, and 10. Significant differences between patients with NAD and AD were calculated using a Student t test (*P < .05; **P < .01; ***P < .001).
Increased plasma levels of TGF-β and TSLP correlate with the increased percentage of CD207 expressing cells in patients with active LCH. (A-B) Plasma levels of TGF-β and TSLP were measured by enzyme-linked immunosorbent assay, and independent data from patients with NAD (N = 12) and AD (N = 9) are graphed. (C-D) Plasma levels of TGF-β and TSLP correlated with the percentage of monocytes (CD11bhighCD11c+) expressing CD207 (CD207+ plus CD207+CD1a+) (Spearman test). (E-F) CD14+ monocytes were isolated from healthy volunteers and cultured in the presence of plasma from patients with AD or NAD (10% vol/vol) for 4 to 10 days. CD207 and CD1a expression was evaluated in CD11c+CD11blow cells by flow cytometry. (E) Representative dot plot of CD207 vs CD1a at day 10. GM-CSF, IL-4, and TGF-β were used as positive controls (see also supplemental Figure 3A). (F) Kinetic expression of CD207 at days 4, 7, and 10. Significant differences between patients with NAD and AD were calculated using a Student t test (*P < .05; **P < .01; ***P < .001).
CD207 and CD1a expression is driven by plasma of patients with active LCH
Because TGF-β and TSLP levels are increased in the plasma of patients with active LCH, and because previous studies have shown that CD14+ monocytes and CD1c blood DCs can attain an LC-like phenotype in response to these cytokines,13-15 we evaluated whether plasma from patients with active LCH could induce CD207+CD1a+ expression on sorted CD14+ monocytes from healthy donors in vitro. Cell phenotype was analyzed at days 4, 7, and 10, and surprisingly, we found significant induction of CD207 on CD11c+CD11blow DCs at day 10 when treated with plasma from patients with active LCH vs those with NAD (Figure 2E-F). Plasma from the same patients, when added to PBMCs, also induced CD207 expression after 7 days in CD11c+CD11blow DCs (supplemental Figure 4B-D). The addition of recombinant GM-CSF, IL-4, and TGF-β on CD14+ monocytes13 resulted in the highest expression of CD207 at day 4 and decreased by day 10 (Figure 2F; supplemental Figure 4A). This difference in kinetics may be due to the use of recombinant cytokines. Our data indicate that a soluble factor from the plasma of patients with active LCH drives the induction of CD207.
This study demonstrates that myeloid CD207+ cells circulate in pediatric patients with AD in correlation with increased levels of TGF-β and TSLP, 2 potential drivers of these LC-like cells. Because this study focused on the pediatric population, further work will need to be performed in order to understand the differences (if any) present in the adult form of LCH. Even though our cohort had few patients, it is important to note that our results show that CD207+ cells in circulation together with TSLP and TGF-β levels could be useful as an indicator of disease activity, can be used to monitor treatment response and disease relapse, and can serve as a predictive biomarker.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to the patients and healthy volunteers who participated in this study. They thank Florencia Quiroga (Instituto de Investigaciones Biomédicas en Retrovirus y SIDA), Plácida Baez, and Ariel Billordo (Instituto de Inmunología, Genética y Metabolismo) for their assistance with flow cytometry (Sistema Nacional de Citometría de Flujo, Argentina), Pamela Chan for editorial assistance with the manuscript, and Martin Krasnapolski (Instituto de Oncología Angel H. Roffo, Universidad de Buenos Aires, Buenos Aires, Argentina) for his immense help and advice during the revision process.
This work was supported by the National Agency for the Promotion of Science and Technology (grant PICTO-GSK 2013-0025) in collaboration with GlaxoSmithKline. L.T. is recipient of the “Alfredo Lanari” doctoral fellowship from Facultad de Medicina, Universidad de Buenos Aires, and C.M.O. received an undergraduate fellowship from the Instituto Nacional del Cáncer, Argentina. E.A.C.S, A.E.E., and D.A.R. are career investigators at the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina.
Authorship
Contribution: E.A.C.S. conceived, designed and performed experiments, analyzed results, and wrote the manuscript; W.N., L.T., C.M.O., J.M.O.W., and I.G.E. performed experiments and analyzed results; G.E. recruited and followed up patients with LCH; A.E.E. conceived, designed and performed experiments, analyzed and discussed results, and wrote the manuscript; D.A.R. recruited and followed up patients with LCH, discussed results, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea Emilse Errasti, Paraguay 2155, 9th Floor, Ciudad Autónoma de Buenos Aires, 1121 Buenos Aires, Argentina; e-mail: andreaerrasti@gmail.com; and Diego Alfredo Rosso, Paraguay 2155, 9th Floor, Ciudad Autónoma de Buenos Aires, 1121 Buenos Aires, Argentina; e-mail: drosso_2000@yahoo.com.
References
Author notes
A.E.E. and D.A.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal