Key Points
After hydroxycarbamide therapy in high-risk ET, ruxolitinib showed no improvement for complete or partial response rates compared with BAT.
Ruxolitinib significantly improved some disease-related symptoms, but rates of thrombosis, hemorrhage, or transformation were not different.
Abstract
Treatments for high-risk essential thrombocythemia (ET) address thrombocytosis, disease-related symptoms, as well as risks of thrombosis, hemorrhage, transformation to myelofibrosis, and leukemia. Patients resistant/intolerant to hydroxycarbamide (HC) have a poor outlook. MAJIC (ISRCTN61925716) is a randomized phase 2 trial of ruxolitinib (JAK1/2 inhibitor) vs best available therapy (BAT) in ET and polycythemia vera patients resistant or intolerant to HC. Here, findings of MAJIC-ET are reported, where the modified intention-to-treat population included 58 and 52 patients randomized to receive ruxolitinib or BAT, respectively. There was no evidence of improvement in complete response within 1 year reported in 27 (46.6%) patients treated with ruxolitinib vs 23 (44.2%) with BAT (P = .40). At 2 years, rates of thrombosis, hemorrhage, and transformation were not significantly different; however, some disease-related symptoms improved in patients receiving ruxolitinib relative to BAT. Molecular responses were uncommon; there were 2 complete molecular responses (CMR) and 1 partial molecular response in CALR-positive ruxolitinib-treated patients. Transformation to myelofibrosis occurred in 1 CMR patient, presumably because of the emergence of a different clone, raising questions about the relevance of CMR in ET patients. Grade 3 and 4 anemia occurred in 19% and 0% of ruxolitinib vs 0% (both grades) in the BAT arm, and grade 3 and 4 thrombocytopenia in 5.2% and 1.7% of ruxolitinib vs 0% (both grades) of BAT-treated patients. Rates of discontinuation or treatment switching did not differ between the 2 trial arms. The MAJIC-ET trial suggests that ruxolitinib is not superior to current second-line treatments for ET. This trial was registered at www.isrctn.com as #ISRCTN61925716.
Introduction
Essential thrombocythemia (ET) is a chronic myeloproliferative neoplasm (MPN) characterized by thrombocytosis. Patients are at higher risk of thrombosis and hemorrhage. They also have disease-related symptoms, which are difficult to manage with standard therapies. Therapeutic approaches address risks of thrombosis and hemorrhage, without increasing transformation into post-ET myelofibrosis (PET-MF) or acute myeloid leukemia (AML).1-3 Low-dose aspirin with hydroxycarbamide (HC) is recommended first-line therapy in high-risk patients, supported by data from randomized trials.3,4 Approximately 20% of ET patients become HC-intolerant or resistant; patients with resistance appear to be at increased risk of disease transformation and reduced overall survival (OS).5 No prospective trial data exist to guide management of ET patients who are HC resistant or intolerant; treatment options are limited, and several second-line treatment options are associated with increased risk of disease transformation.2,3,6
The discovery of the Janus kinase (JAK2V617F) mutation provided the first genetic marker of the malignant clone in MPN.7-9 Furthermore, other key driver mutations associated with ET, affecting thrombopoietin receptor MPL and calreticulin (CALR), also led to increased JAK2 signaling.10 The JAK1/2 inhibitor, ruxolitinib, was effective in reducing spleen volume, controlling blood counts, and improving symptoms in MF and polycythemia vera (PV) patients.11-13 Ruxolitinib treatment may also result in a survival advantage for patients with MF.14,15 A previous nonrandomized study in 39 ET patients, resistant or intolerant to HC, demonstrated that ruxolitinib lowered both platelet and white cell counts, and the most effective starting dose was 25 mg twice daily.16
We conducted a randomized, phase 2 trial to evaluate the activity and safety of ruxolitinib vs best available therapy (BAT) in 2 different patient populations (ET and PV): a randomized study of BAT vs JAK inhibition in patients with high-risk PV or ET who are resistant or intolerant to HydroxyCabamide (MAJIC). The study used an efficient framework of a basket trial design, permitting the separate evaluation of 2 study populations. Here, we present safety and efficacy data for the ET population, so-called MAJIC-ET.
Patients and methods
Trial design
An independent, parallel, open-label, randomized controlled trial of ruxolitinib vs BAT was implemented (supplemental Figure 1, available on the Blood Web site). Patients aged ≥18 with high-risk ET or PV, who met modified criteria for intolerance or resistance to HC17 (supplemental Table 1), were recruited. The MAJIC-PV arm is on-going. High-risk ET was defined by standard criteria (supplemental Table 2). Patients were stratified by JAK2V617F status and randomized 1:1 to receive either ruxolitinib (starting dose 25 mg twice daily or 20 mg twice daily, if baseline platelets were 100 to 200 × 109/L) or BAT. Inclusion and exclusion criteria are presented (supplemental Table 3). The trial was registered at www.isrctn.com (ISRCTN61925716) and reviewed by an independent research ethics committee. All participants gave written informed consent in accordance with the Declaration of Helsinki. Trial data were analyzed by statisticians at the Cancer Research UK Clinical Trials Unit (University of Birmingham), and quality of life (QoL) analysis was performed by statisticians at the Mayo Clinic, Scottsdale and Phoenix, Arizona. Ruxolitinib was provided free of charge by Novartis. All authors had access to primary clinical trials data and approved the final version of the manuscript.
Outcome measures
The primary outcome measure was achievement of complete response (CR) as defined by European LeukemiaNet (ELN) criteria within 1 year of treatment.18 CR in ET patients was defined by achieving all of the following criteria: platelet count ≤400 × 109/L; normal spleen size on imaging; white blood cell count ≤10 × 109/L. Secondary outcomes included partial response (PR) per ELN criteria within 1 year of treatment, duration of response (both CR and PR) and overall response (ie, CR and PR), toxicity profile of ruxolitinib based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4, dose intensity, histological response, molecular response (MR), hemorrhagic and thromboembolic events, disease transformations, QoL and disease symptom burden, and overall and progression-free survival (PFS). The safety population included all patients who received at least 1 dose of protocol treatment. Hemorrhagic and thrombotic events were centrally reviewed. QoL and symptom assessment questionnaires: 10-item Myeloproliferative Neoplasm Symptom Assessment Form total symptom score (TSS),19 EQ-5D (a standardized instrument for measuring generic health status),20 and MD Anderson Symptom Inventory (MDASI),21 were completed at baseline (pretreatment, 7 consecutive days for the 10-item Myeloproliferative Neoplasm Symptom Assessment Form, and once for the other questionnaires), and at 2 and 4 months postrandomization, and continued every 4 months while on trial. Overall symptom response was defined as at least a 50% reduction in TSS from baseline (average of the 7 baseline days with at least 4 of 7 days scored) at any postbaseline time point up to month 12.
Sample size justification and statistical analysis
Sample size calculations were based upon rates from a previous phase 2 study16 using a 1-sided normal test without continuity correction and unpooled variance. CR rate for controls was estimated at 30%. A clinically significant improvement was considered to be 20%. Thus, assuming CR rates in the control and treatment group were 30% and 50%, 55 patients were required in each arm to detect a clinically significant difference of 20% with 82% statistical power at 10% level of significance. Because this is a randomized screening trial to evaluate a direct, but nondefinitive comparison, with the aim of screening for promising signal of activity in ruxolitinib, a relaxed 1-sided significance level of 10% was used.22 Allowing for a 5% dropout rate, 116 patients were required.
P < .10 was considered significant for the primary outcome. For other analyses, 2-sided tests were used, and P < .05 was considered significant. Number and proportion of patients were reported for categorical variables by treatment group and overall. Descriptive statistics (number of patients, mean, standard deviation [SD], median, interquartile range) were reported for continuous variables by treatment group and overall. Time-to-event outcomes were analyzed using the method of Kaplan and Meier, and differences in survival time were determined using a Cox’s model with an adjustment for JAK2V617F status per baseline data. Sensitivity analysis adjusting the Cox models for hemoglobin and disease duration was performed and reported where treatment effect differed. Normal z tests were used to assess differences in proportions. Univariate and multivariate logistic regression were fitted models to assess the effect of baseline measures on primary outcome, transformations, and toxicity. Apart from the primary outcome, additional hypotheses testing were exploratory and not prespecified. All summaries and statistical analyses for efficacy were primarily carried out on a modified intention-to-treat (mITT) basis, including patients analyzed according to their randomized treatment allocation, starting treatment within 1 year of randomization, and with at least 1 response. Summary statistics for safety variables were based on the safety population, which included patients according to the treatment they actually received and who received >1 doses of treatment. Statistical analyses were performed using Stata version 14.2.
QoL and symptom data were analyzed in the mITT population using SAS version 4. Symptom response rate was compared using the χ2 test, with maximum percentage reduction from baseline during the first 12 months using a Wilcoxon rank-sum test. Symptom response and percentage reduction at postbaseline time points (or at most recent assessment if no symptom data at the given time point were provided) were compared using χ2 and Wilcoxon rank-sum tests. Comparisons of mean scores longitudinally employed a linear mixed model for each outcome (from month 2 assessment) using all available data. In addition, each model included a continuous covariate for baseline value of outcome and used planned month of assessment as categorical time value with compound covariance structure.
Treatment and assessments
Ruxolitinib was initiated based on baseline platelet count. BAT was assigned according to physician’s choice but had to be an active agent; change of and combination of BAT therapies were permitted with the aim of achieving a CR. No crossover of BAT to ruxolitinib was permitted. Low-dose aspirin (75 mg once daily) was advised unless contraindicated. Protocol-specified dose reductions for ruxolitinib were in place, and patients were allowed to reescalate if toxicity had resolved. Lowest permitted dose of ruxolitinib was 5 mg once daily. Hematological response was assessed every 2 weeks for 3 months and every 6 weeks thereafter in order to determine the primary outcome of CR during year 1 (cutoff week 54). Ultrasound was performed at baseline and centrally reviewed. If splenomegaly was present at baseline, repeat ultrasound showing resolution was required for CR. Ruxolitinib continued beyond 1 year if CR or PR was maintained. Those discontinuing ruxolitinib moved to the BAT arm for follow-up. Patients who transformed to PET-MF, myelodysplastic syndrome, or AML discontinued the trial but were followed for survival.
Assays for JAK2V617F, CALR, and MPL mutation allele burden were quantified using next-generation sequencing as previously described.23 An analysis of histological features is currently being performed, and these data are not being presented as part of this article.
Results
Patient characteristics
One hundred sixteen patients were recruited in 31 UK centers between September 2012 and February 2015, with median follow-up of 2.61 years (range: 0.23-4.12). In total, 110 were eligible for the mITT analysis, comprising 58 (52%) and 52 (48%) patients in the ruxolitinib and BAT arms, respectively. Median age of patients was 64.2 years with 44 (40%) male and 66 (60%) female patients; overall 28/110 (25.4%) were resistant to HC, 57/110 (51.8%) intolerant, and 25/110 (22.7%) were both. Baseline characteristics were balanced (Table 1), except for the ruxolitinib arm with longer disease duration and lower hemoglobin. Six patients were excluded from mITT analysis: 4 withdrew without treatment (2 did not wish to be on the BAT arm, 1 was ineligible, 1 had transformed to PET-MF) and 2 did not start treatment within 1 year from randomization. All CALR indels and MPL mutations are provided in supplemental Table 4.
Baseline characteristics by treatment
| . | BAT (n = 52) . | Ruxolitinib (n = 58) . | Overall (N = 110) . |
|---|---|---|---|
| Age, y | |||
| Mean (SD) [range] | 65.6 (13.5) [37.2, 85.4] | 62.9 (12.3) [34.5, 90.5] | 64.2 (12.9) [34.5, 90.5] |
| Sex, n (%) | |||
| Female | 30 (57.7) | 36 (62.1) | 66 (60.0) |
| Male | 22 (42.3) | 22 (37.9) | 44 (40.0) |
| Mutation status, n (%) | |||
| JAK2V617F positive | 26 (50.0) | 28 (48.3) | 54 (49.1) |
| CALR mutation positive | 14 (26.9) | 20 (34.5) | 34 (30.9) |
| MPL mutation positive | 3 (5.8) | 3 (5.2) | 6 (5.5) |
| Triple negative | 7 (13.5) | 6 (10.3) | 13 (11.8) |
| Not run | 2 (3.8) | 1 (1.7) | 3 (2.7) |
| HC resistant or intolerant,*n (%) | |||
| Resistant | 25 (48.1) | 28 (48.3) | 53 (48.2) |
| Intolerant | 27 (51.9) | 30 (51.7) | 57 (51.8) |
| Time from diagnosis to randomization, y† | |||
| Mean (SD) [range] | 6.9 (5.8) [0.4, 23.6] | 10.4 (6.7) [0.7, 31.2] | 8.8 (6.5) [0.4, 31.2] |
| Hemoglobin, g/L† | |||
| Mean (SD) [range] | 126 (17) [90.0, 160.0] | 119 (17) [87.0, 152.0] | 122 (17) [87.0, 160.0] |
| Platelet count, ×109/L | |||
| Mean (SD) [range] | 573.0 (227.1) [166.0, 1406.0] | 545.4 (215.3) [89.0, 1139.0] | 558.4 (220.4) [89.0, 1406.0] |
| WBC count, ×109/L | |||
| Mean (SD) [range] | 6.8 (2.7) [2.8, 15.2] | 7.5 (4.8) [1.7, 29.8] | 7.2 (3.9) [1.7, 29.8] |
| Hematocrit | |||
| Mean (SD) [range] | 0.4 (0.1) [0.3, 0.5] | 0.4 (0.1) [0.3, 0.5] | 0.4 (0.1) [0.3, 0.5] |
| Spleen size | |||
| Enlarged | 9 | 14 | 23 |
| Normal | 38 | 37 | 75 |
| Splenectomy | 2 | 3 | 5 |
| Missing | 3 | 4 | 7 |
| Number of previous therapies, n (%) | |||
| 1 | 15 (28.8) | 14 (24.1) | 28 (26.4) |
| 2 | 20 (38.5) | 24 (41.4) | 44 (40.0) |
| 3 | 8 (15.4) | 12 (20.7) | 20 (18.2) |
| 4 | 5 (9.6) | 5 (8.6) | 10 (9.1) |
| 5 | 2 (3.8) | 2 (3.4) | 4 (3.6) |
| 6 | 2 (3.8) | 0 (0) | 2 (1.8) |
| 9 | 0 (0) | 1 (1.7) | 1 (0.9) |
| Total number of previous therapies by treatment, n (%)‡ | |||
| HC | 59 (52.2) | 70 (58.8) | 129 (55.6) |
| Anagrelide | 29 (25.7) | 31 (26.1) | 60 (25.9) |
| Interferon | 7 (6.2) | 11 (9.2) | 18 (7.8) |
| Pegylated interferon | 2 (1.8) | 2 (1.7) | 4 (1.7) |
| Busulfan | 8 (7.1) | 1 (0.8) | 9 (3.9) |
| 32P | 3 (2.7) | 1 (0.8) | 4 (1.7) |
| Pipobroman | 1 (0.9) | 1 (0.8) | 2 (.09) |
| Fedratinib | 1 (0.9) | 1 (0.8) | 2 (0.9) |
| Vorinostat | 2 (1.8) | 0 (0) | 2 (0.9) |
| Thalidomide | 0 (0) | 1 (0.8) | 1 (0.4) |
| Missing | 1 (0.9) | 0 (0) | 1 (0.4) |
| . | BAT (n = 52) . | Ruxolitinib (n = 58) . | Overall (N = 110) . |
|---|---|---|---|
| Age, y | |||
| Mean (SD) [range] | 65.6 (13.5) [37.2, 85.4] | 62.9 (12.3) [34.5, 90.5] | 64.2 (12.9) [34.5, 90.5] |
| Sex, n (%) | |||
| Female | 30 (57.7) | 36 (62.1) | 66 (60.0) |
| Male | 22 (42.3) | 22 (37.9) | 44 (40.0) |
| Mutation status, n (%) | |||
| JAK2V617F positive | 26 (50.0) | 28 (48.3) | 54 (49.1) |
| CALR mutation positive | 14 (26.9) | 20 (34.5) | 34 (30.9) |
| MPL mutation positive | 3 (5.8) | 3 (5.2) | 6 (5.5) |
| Triple negative | 7 (13.5) | 6 (10.3) | 13 (11.8) |
| Not run | 2 (3.8) | 1 (1.7) | 3 (2.7) |
| HC resistant or intolerant,*n (%) | |||
| Resistant | 25 (48.1) | 28 (48.3) | 53 (48.2) |
| Intolerant | 27 (51.9) | 30 (51.7) | 57 (51.8) |
| Time from diagnosis to randomization, y† | |||
| Mean (SD) [range] | 6.9 (5.8) [0.4, 23.6] | 10.4 (6.7) [0.7, 31.2] | 8.8 (6.5) [0.4, 31.2] |
| Hemoglobin, g/L† | |||
| Mean (SD) [range] | 126 (17) [90.0, 160.0] | 119 (17) [87.0, 152.0] | 122 (17) [87.0, 160.0] |
| Platelet count, ×109/L | |||
| Mean (SD) [range] | 573.0 (227.1) [166.0, 1406.0] | 545.4 (215.3) [89.0, 1139.0] | 558.4 (220.4) [89.0, 1406.0] |
| WBC count, ×109/L | |||
| Mean (SD) [range] | 6.8 (2.7) [2.8, 15.2] | 7.5 (4.8) [1.7, 29.8] | 7.2 (3.9) [1.7, 29.8] |
| Hematocrit | |||
| Mean (SD) [range] | 0.4 (0.1) [0.3, 0.5] | 0.4 (0.1) [0.3, 0.5] | 0.4 (0.1) [0.3, 0.5] |
| Spleen size | |||
| Enlarged | 9 | 14 | 23 |
| Normal | 38 | 37 | 75 |
| Splenectomy | 2 | 3 | 5 |
| Missing | 3 | 4 | 7 |
| Number of previous therapies, n (%) | |||
| 1 | 15 (28.8) | 14 (24.1) | 28 (26.4) |
| 2 | 20 (38.5) | 24 (41.4) | 44 (40.0) |
| 3 | 8 (15.4) | 12 (20.7) | 20 (18.2) |
| 4 | 5 (9.6) | 5 (8.6) | 10 (9.1) |
| 5 | 2 (3.8) | 2 (3.4) | 4 (3.6) |
| 6 | 2 (3.8) | 0 (0) | 2 (1.8) |
| 9 | 0 (0) | 1 (1.7) | 1 (0.9) |
| Total number of previous therapies by treatment, n (%)‡ | |||
| HC | 59 (52.2) | 70 (58.8) | 129 (55.6) |
| Anagrelide | 29 (25.7) | 31 (26.1) | 60 (25.9) |
| Interferon | 7 (6.2) | 11 (9.2) | 18 (7.8) |
| Pegylated interferon | 2 (1.8) | 2 (1.7) | 4 (1.7) |
| Busulfan | 8 (7.1) | 1 (0.8) | 9 (3.9) |
| 32P | 3 (2.7) | 1 (0.8) | 4 (1.7) |
| Pipobroman | 1 (0.9) | 1 (0.8) | 2 (.09) |
| Fedratinib | 1 (0.9) | 1 (0.8) | 2 (0.9) |
| Vorinostat | 2 (1.8) | 0 (0) | 2 (0.9) |
| Thalidomide | 0 (0) | 1 (0.8) | 1 (0.4) |
| Missing | 1 (0.9) | 0 (0) | 1 (0.4) |
WBC, white blood cell.
Twenty-five patients were both resistant and intolerant. These patients have been included as resistant.
Time from diagnosis to randomization and baseline hemoglobin were different between the 2 treatment arms.
Patients were allowed to receive multiple therapies; therefore, total number of therapies in each category might exceed number of patients.
Trial treatment
For patients receiving ruxolitinib, the mean dose intensity of ruxolitinib during year 1 was 19 mg twice daily (Figure 1). The most common BAT therapies used at least once included HC in 37/52 (71.1%), anagrelide in 25/52 (48.1%), and interferon in 21/52 (40.4%) patients.
Doses of ruxolitinib throughout the MAJIC-ET trial. This figure shows the mean dose of ruxolitinib throughout the MAJIC-ET trial. The mean dose intensity for ruxolitinib during year 1 was 19 mg twice daily.
Doses of ruxolitinib throughout the MAJIC-ET trial. This figure shows the mean dose of ruxolitinib throughout the MAJIC-ET trial. The mean dose intensity for ruxolitinib during year 1 was 19 mg twice daily.
Patient disposition
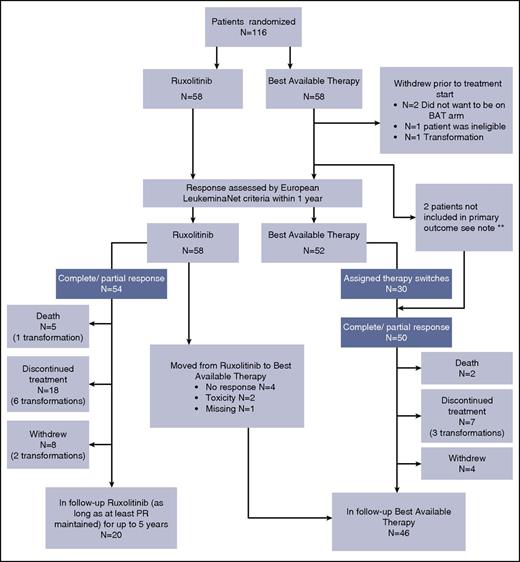
Patient disposition at the time of analysis (2-year follow-up) is shown in Figure 2. Thirty BAT patients (57.7%) switched their initially assigned therapy at least once, and there were 86 switches across the BAT group. In total, 45 patients (49.5%) discontinued treatment, with 40 discontinuations occurring within the first treatment year. Thirty-five patients (60.3%) receiving ruxolitinib and 10 patients (19.2%) receiving BAT discontinued treatment. The main reasons for discontinuation in the ruxolitinib arm were loss of response (11/35 [31.4%]) and transformation (9/35 [25.7%]). The main reasons for discontinuation in the BAT arm were transformation (3/10 [30%]) and death (2/10 [20%]). Discontinuations and therapy switches are shown in Table 2.
Trial consort diagram at 2-year follow-up. Death was recorded as such regardless of prior discontinuation or withdrawal. Patients were clustered as withdrawals regardless of prior discontinuations. **Note: 1 BAT patient started treatment 1 year after randomization; 1 other BAT patient did not have a recorded response within 1 year. Both have been excluded from primary analysis, but included in the follow-up.
Trial consort diagram at 2-year follow-up. Death was recorded as such regardless of prior discontinuation or withdrawal. Patients were clustered as withdrawals regardless of prior discontinuations. **Note: 1 BAT patient started treatment 1 year after randomization; 1 other BAT patient did not have a recorded response within 1 year. Both have been excluded from primary analysis, but included in the follow-up.
Overview of assigned therapy switches and discontinuations per treatment arm
| . | Ruxolitinib . | BAT . | Total . |
|---|---|---|---|
| Assigned therapy switches | |||
| Patients that switched BAT therapy at least once | N/A | 30 | 30 |
| Total number of times BAT therapy was switched | N/A | 86 | 86 |
| Discontinuations | |||
| Transformation | 9 | 3 | 12 |
| Loss of response | 11 | 0 | 11 |
| Lack of efficacy | 5 | 1 | 6 |
| Toxicity | |||
| Anemia | 2 | 0 | 2 |
| Other | 3 | 1 | 4 |
| Other | 3 | 3 | 6 |
| Death | 1 | 2 | 3 |
| Withdrawal of consent | 1 | 0 | 1 |
| Total | 35 | 10 | 45 |
| . | Ruxolitinib . | BAT . | Total . |
|---|---|---|---|
| Assigned therapy switches | |||
| Patients that switched BAT therapy at least once | N/A | 30 | 30 |
| Total number of times BAT therapy was switched | N/A | 86 | 86 |
| Discontinuations | |||
| Transformation | 9 | 3 | 12 |
| Loss of response | 11 | 0 | 11 |
| Lack of efficacy | 5 | 1 | 6 |
| Toxicity | |||
| Anemia | 2 | 0 | 2 |
| Other | 3 | 1 | 4 |
| Other | 3 | 3 | 6 |
| Death | 1 | 2 | 3 |
| Withdrawal of consent | 1 | 0 | 1 |
| Total | 35 | 10 | 45 |
N/A, not applicable.
Efficacy analysis
For patients meeting the criteria for mITT analysis, the primary outcome (CR) was achieved in 27 (46.5%) patients in the ruxolitinib arm vs 23 (44.2%) patients in the BAT arm (unadjusted P = .40, adjusted for JAK2V617F status P = .40) with a difference of proportions −2.3% between BAT and ruxolitinb (80% confidence interval [CI]: −15%, 10%). PR occurred in 27 (46.5%) patients in the ruxolitinib arm and 27 (51.9%) patients in the BAT arm. Time to first response (CR or PR) between the 2 arms was significantly different (P = .01) with BAT patients taking longer. Duration of CR appeared shorter for ruxolitinib patients (borderline significant difference, P = .05; adjustment for hemoglobin and disease duration rendered this insignificant, P = .2). There was no evidence of a difference in duration of overall response between ruxolitinib and BAT. OS and PFS at 1 year were similar (OS: 0.98 [95% CI 0.86, 0.99] for BAT and 0.98 [95% CI 0.88, 0.99] for ruxolitinib patients, PFS: 0.96 [95% CI 0.85, 0.99] for BAT and 0.93 [95% CI 0.81, 0.97] for ruxolitinib patients). In multivariable analyses performed to assess baseline factors influencing CR (modeled for treatment received, HC resistance/intolerance, white cell count, platelets, hemoglobin, and JAK2/CALR status), no factor was shown to be significant and did not change the treatment effect (supplemental Table 5).
Thrombosis, hemorrhage, and disease transformation
After 2 years of follow-up, transformation to PET-MF occurred in 8 ruxolitinib- vs 5 BAT-treated patients. Transformation to AML was seen in 1 patient who received ruxolitinib. Transformation-free probability was not significantly different between the 2 arms (P = .29; supplemental Figure 2A). Concerning thrombosis and hemorrhage, following central review, 10 patients (17.2%) on the ruxolitinib arm experienced 11 thrombotic events compared with 3 patients (5.8%) on the BAT arm, who experienced 5 events. Hemorrhagic events were 1 (1.7%) vs 5 (8.9%) for ruxolitinib and BAT patients, respectively (Table 3). Concerning thrombosis-free probability, the differences were borderline but not statistically significant (P = .09; supplemental Figure 2B). Hemorrhage was less frequent for patients treated with ruxolitinib; however, this difference was also not significant (P = .14; supplemental Figure 2C). Because all of these events are considered clinically relevant, we performed an analysis of transformation, thrombosis, and hemorrhage as a composite endpoint; there was no evidence of a difference (P = .35; supplemental Figure 2D). Most thrombotic and hemorrhagic events occurred in patients in CR or PR (supplemental Figure 3). In a multivariate analysis of factors influencing transformation to PET-MF, this event only occurred in patients with baseline WBC < 10 × 109/L (supplemental Table 6).
Thrombotic and hemorrhagic events
| . | . | BAT . | Ruxolitinib . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Grade 1 and 2 . | Grade 3 and 4 . | Grade 5 . | Grade 1 and 2 . | Grade 3 and 4 . | Grade 5 . | ||
| Hemorrhagic events | ||||||||
| Hematuria | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Intracranial hemorrhage | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Oral hemorrhage | 1 | 0 | 0 | 1 | 0 | 0 | 2 | |
| Rectal hemorrhage | 1 | 1 | 0 | 0 | 0 | 0 | 2 | |
| Total | 3 | 1 | 1 | 1 | 0 | 0 | 6 | |
| Thrombotic events* | ||||||||
| Chest pain, cardiac | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Myocardial infarction | 0 | 0 | 0 | 0 | 2 | 0 | 2 | |
| Cerebrovascular ischemia | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Retinal vascular disorder | 1 | 0 | 0 | 1 | 0 | 0 | 2 | |
| Thromboembolic events | ||||||||
| PE | 0 | 0 | 0 | 0 | 3† | 0 | 3 | |
| DVT | 0 | 0 | 0 | 1 | 1 | 0 | 2 | |
| Calf vein DVT | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Transient ischemic attacks | 2 | 0 | 0 | 2 | 0 | 0 | 4 | |
| Total | 4 | 1 | 0 | 4 | 7 | 0 | 16 | |
| . | . | BAT . | Ruxolitinib . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Grade 1 and 2 . | Grade 3 and 4 . | Grade 5 . | Grade 1 and 2 . | Grade 3 and 4 . | Grade 5 . | ||
| Hemorrhagic events | ||||||||
| Hematuria | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Intracranial hemorrhage | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Oral hemorrhage | 1 | 0 | 0 | 1 | 0 | 0 | 2 | |
| Rectal hemorrhage | 1 | 1 | 0 | 0 | 0 | 0 | 2 | |
| Total | 3 | 1 | 1 | 1 | 0 | 0 | 6 | |
| Thrombotic events* | ||||||||
| Chest pain, cardiac | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Myocardial infarction | 0 | 0 | 0 | 0 | 2 | 0 | 2 | |
| Cerebrovascular ischemia | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Retinal vascular disorder | 1 | 0 | 0 | 1 | 0 | 0 | 2 | |
| Thromboembolic events | ||||||||
| PE | 0 | 0 | 0 | 0 | 3† | 0 | 3 | |
| DVT | 0 | 0 | 0 | 1 | 1 | 0 | 2 | |
| Calf vein DVT | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Transient ischemic attacks | 2 | 0 | 0 | 2 | 0 | 0 | 4 | |
| Total | 4 | 1 | 0 | 4 | 7 | 0 | 16 | |
DVT, deep vein thrombosis; PE, pulmonary embolism.
The death of a ruxolitinib treated patient due to ischemic cardiomyopathy occurred >30 d past treatment and is therefore not recorded as an event.
One patient experienced PE and DVT at the same time, but was counted in the PE category.
Molecular responses
The mean baseline allele burdens for JAK2V617F, CALR, or MPL mutation-positive patients are displayed in Table 1. At 12 months, or the last available sample during year 1, the overall mean allele burden had not changed significantly for any mutation in either treatment arm. However, 1 complete molecular response (CMR) and 1 partial molecular response (PMR) per ELN criteria were seen for JAK2V617F-positive patients on the ruxolitinib arm and 2 CMRs and 1 PMR for CALR-positive patients on ruxolitinib compared with 0 CMRs/PMRs for patients with these mutations receiving BAT. A JAK2V617F-positive patient who achieved a PMR on ruxolitinib also had resolution of a cytogenetic abnormality at 1 year. There was no pattern of MR or progression with complete or partial hematological response or transformation, but 1 CALR-positive patient who transformed to PET-MF had a CMR.
Impact on ET-related disease symptom burden
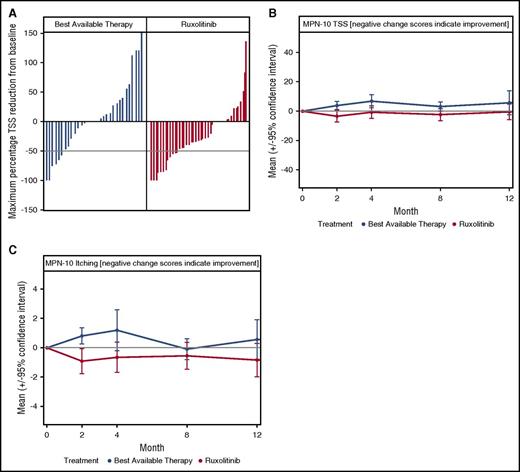
Among 110 patients in the mITT cohort, 85 completed the baseline and at least 1 postbaseline questionnaire (ruxolitinib N = 47, BAT N = 38). Although overall symptom response rate during the first 12 months did not significantly differ between arms (ruxolitinib 12/42 [29%] vs BAT 6/31 [19%], P = .37), maximum percentage TSS reduction at any point during the first 12 months of treatment was significantly greater for ruxolitinib compared with BAT (median reduction 32% vs 0%, P = .03; Figure 3A). Symptom response was rapid in the ruxolitinib arm (8/42 [19%] at 2 months) as compared with BAT (1/31 [3%] at 2 months, P = .04). Longitudinally, mean TSS (P = .03) and the individual symptom of pruritus (P = .01) were significantly lower for ruxolitinib vs BAT (Figure 3B-C), with trends observed for improved concentration (P = .05), lower anxiety/depression (EQ-5D, P = .09), and higher ability to perform usual activities (EQ-5D, P = .09) on the ruxolitinib arm compared with BAT.
Changes in ET-related symptom burden during year 1 of the MAJIC-ET trial. (A) A waterfall plot of maximum percentage change in the MPN-10 TSS score; dotted line indicates 50% reduction in TSS. (B) The mean MPN-10 TSS throughout the first year of the trial; there was a consistent trend for reduction for ruxolitinib. (C) The mean MPN-10 score for itching during the first 12 months of the MAJIC-ET trial.
Changes in ET-related symptom burden during year 1 of the MAJIC-ET trial. (A) A waterfall plot of maximum percentage change in the MPN-10 TSS score; dotted line indicates 50% reduction in TSS. (B) The mean MPN-10 TSS throughout the first year of the trial; there was a consistent trend for reduction for ruxolitinib. (C) The mean MPN-10 score for itching during the first 12 months of the MAJIC-ET trial.
Safety
All safety analysis was conducted on the safety population: 115 patients (57 BAT, 58 ruxolitinib). A total of 128 grade 3/4 events occurred in 89 patients on the trial (supplemental Table 7). Hematological toxicities (36/128) and metabolism/nutrition disorders (17/128, 10 relating to hyponatremia) were the most common. Grade 3 or 4 anemia occurred in 12/58 (21%) of ruxolitinib patients vs 0/57 (0%) in the BAT patients (P < .005), grade 3 or 4 thrombocytopenia in 2/58 (3.4%) of ruxolitinib vs 0/57 (0%) of BAT patients (P = .32), and grade 3 (only) infections occurred in 9/58 (15.5%) of patients in the ruxolitinib arm compared with 2/57 (3.5%) (grade 3 and 4) in the BAT arm (P = .03). Overall, 2 patients discontinued ruxolitinib for anemia; there were no discontinuations related to thrombocytopenia. Blood counts during the trial according to treatment arm are shown in supplemental Figure 4, demonstrating equivalent control of leukocytes and platelets but lower hemoglobin from week 4. An unplanned multivariate model (modeled for hemoglobin [≥100 g/dL] and JAK2/CALR status) demonstrated that baseline hemoglobin (≥100 g/dL) was significant in predicting the occurrence of anemia or thrombocytopenia (odds ratio = 0.17, 95% CI = 0.04, 0.72, P = .01) (supplemental Table 8).
There were 5 patient deaths in the ruxolitinib arm and 2 in the BAT arm; none were considered treatment related. The deaths in the BAT arm were due to multiple organ failure and cerebral hemorrhage. In the ruxolitinib arm, deaths were due to carcinomatosis combined with esophageal cancer, bowel infarction due to adhesions, acute left ventricular failure, ischemic cardiomyopathy, and sepsis combined with pancreatic cancer.
Discussion
ET is often regarded as the most indolent of the Philadelphia-negative MPNs; treatments offer improvements in blood counts and reduction in risk of thrombosis and hemorrhage with a lack of certainty regarding effects upon transformation to PET-MF and AML.2,3 Criteria for resistance or intolerance to therapy with HC were originally developed to guide clinicians when to initiate second-line therapies; however, there is now evidence that HC-resistant patients have a poor outlook.5 In addition, disease-related symptom burden is increasingly recognized as an important disease feature, causing significant morbidity with few effective treatments.2-4 In previous studies, patients with MF gained a survival benefit with ruxolitinib, which also reduced spleen size and symptoms compared with BAT.14 In the RESPONSE study in HC-resistant/intolerant PV patients, there was a suggestion of lower rates of thrombosis in patients receiving ruxolitinib compared with BAT as well as better control of blood counts, spleen size, and symptoms.13
The MAJIC trial was designed to compare ruxolitinib with BAT in patients with HC intolerance/ resistance in 2 populations, MAJIC-ET and MAJIC-PV. Both trial populations are fully recruited, and here we report the findings of the MAJIC-ET trial. The patients recruited into MAJIC-ET displayed characteristics that were well balanced between the 2 arms with the exception of baseline hemoglobin and prior disease duration. Distribution of driver mutations, JAK2V617F, MPL exon 10, and CALR mutations were as expected. Our patients had a long disease duration (up to 31 years), some of whom had received multiple therapy lines with up to 9 prior therapies. Some features of advanced disease, for example, splenomegaly and leukocytosis, were present at baseline; however, transformation to PET-MF was excluded at trial entry. Diagnostic criteria have been controversial in ET, and those in use at trial centers were British Committee for Standards in Hematology (BCSH; n = 18); World Health Organization (WHO) 2001/2008 (n = 10), and both combined (n = 3). The BCSH and WHO criteria were recently shown to perform equally well.24
Usual therapy choice in the second-line setting for ET would be anagrelide or interferon; however, in order to perform a “real-life comparison,” we allowed investigator choice. Overall, the majority, 79% (41/52), of BAT patients received 1 or both agents before or during the study. On-study BAT included in addition busulfan 32P and HC; several international guidelines recommend busulfan or 32P for older patients.
Proportions of patients reaching CR within 1 year were similar: 27 (46.5%) in the ruxolitinib arm vs 23 (44.2%) for BAT, with similar PR rates. Time to any first response (CR or PR) was significantly faster for patients treated with ruxolitinib (P = .01). A particularly interesting finding was that patients in CR who were randomized to receive ruxolitinib had to change therapy and potentially lost any preexisting response yet managed to attain CR faster than BAT patients who may not have changed therapy, thus only needing to maintain response. In addition, BAT patients were also allowed to combine or to switch therapies and frequently did so. Importantly, the duration of CR appeared shorter for ruxolitinib patients with a marginally significant value, whereas the duration of overall response (CR and PR) was not different between both arms. We confirm that HC-resistant/intolerant ET patients have a high risk of thrombosis, hemorrhage, and transformation to PET-MF, event rates here being higher than reported in the nonresistant/intolerant patients (eg, PT-1 or ANAHYDRET studies).25,26 However, overall thrombosis, hemorrhage, or transformation considered separately or together as a composite endpoint was not statistically different between the ruxolitinib and BAT. Furthermore, in a post hoc unplanned analysis for factor influencing transformation to PET-MF, only a leukocyte count <10 × 109/L was significant.
Studies have reported that postrandomization exclusions of patients in randomized trials may affect trial results,27 with some raising concerns that the investigated therapy might be favored.28,29 However, in the MAJIC-ET trial, if we were to conduct a pure intention-to-treat (ITT) analysis, this would require imputation of missing response data for 6 BAT patients. Missing data imputation may bias estimates of treatment effects.30 A commonly used technique is nonresponder imputation, which will attribute all 6 BAT patients as not achieving CR within a year. Utilizing nonresponder imputation resulted in a less conservative ITT analysis of 23/58 CR (BAT) vs 27/58 CR, with P value of .22 compared with the mITT analysis (P = .4). Our primary findings of no evidence of superiority of ruxolitinib were however consistent using either mITT or ITT analysis.
MRs were uncommon in the first year of the trial, as described previously.16 However, ruxolitinib was associated with 2 CMR and 1 PMR in a CALR-positive patient; this has not previously been reported. Transformation to PET-MF in 1 CALR-positive patient, who achieved a CMR, presumably occurred because of the emergence of a different clone, consistent with patients reported with JAK2V617F-positive chronic phase developing JAK2V617F-negative AML,31 and raises questions about the relevance and value of CMR in patients with ET.
Patterns of adverse events with ruxolitinib were similar to those already reported, most prevalent events related to hematological, nutritional, and metabolic events. Infections were also more common with ruxolitinib therapy. There was no suggestion of imbalance between the 2 arms of MAJIC-ET for non–melanoma skin cancer as was previously noted in the RESPONSE trial.13 Treatment discontinuation occurred more frequently for patients treated with ruxolitinib, with 35 patients discontinuing treatment compared with 9 discontinuations in the BAT arm. However, 30 BAT patients switched their initially assigned BAT treatment for various reasons, which indicates a similar rate of treatment ineffectiveness or intolerance. For the first time, we show baseline anemia predicted for treatment-emergent anemia and thrombocytopenia.
Patients with ET have a high burden of symptoms, which have been consistently reported to affect their QoL.32 The symptom response rate, defined as a 50% reduction in TSS, during the first 12 months did not significantly differ between the 2 arms. However, maximum percentage TSS reduction during the first 12 months of treatment was significantly greater for ruxolitinib compared with BAT and was more rapid in the ruxolitinib arm. Longitudinally, mean TSS and individual symptoms of pruritus were significantly lower for ruxolitinib, with trends observed for improved concentration, lower anxiety/depression, and higher ability to perform usual activities for ruxolitinib arm compared with BAT, indicating a novel and important benefit to ET patients of ruxolitinib therapy.
Limitations of our trial include that the trial reflected “real-life practice” in use of diagnostic criteria and selection of BAT therapies. The majority of our centers used either BCSH (n = 18) or WHO (n = 10) or both (n = 3) diagnostic criteria, thus perhaps illustrating nonstandardized diagnostic processes; however, this is a second-line study and BCSH/WHO criteria both perform equally well.24 In addition, transformation was excluded at study entry. Guidelines recommend anagrelide or interferon as second-line therapy for ET. Many BAT patients had already been treated with these drugs (25 received interferon, 7 anagrelide, and 7 both agents) before study entry, and overall, 79% received them before or during the study. The use of HC as a BAT and frequent switching of BAT therapies in 30 BAT patients also reflect real-life constraints and limited treatment options for ET patients with resistance/intolerance to HC and highlight the need for newer therapies in this field.
In conclusion, the MAJIC-ET trial suggests that ruxolitinib does not have improved treatment efficacy compared with BAT for most clinically relevant events. Symptom responses were superior with ruxolitinib therapy, but there was no difference in this study for control of blood counts or other relevant endpoints, such as transformation, thrombosis, or hemorrhage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge the following Principal Investigators and their teams for their contribution to the trial: Henry Watson (Aberdeen Royal Infirmary), Anna Godfrey (Addenbrooke's Hospital), David Galvani (Arrowe Park Hospital), Mark Drummond (Beatson West of Scotland Cancer Centre), Tim Somervaille (Christie Hospital), Raphael Ezekwesili (Darent Valley Hospital), Jonathan Wallis (Freeman Hospital), Rebecca Frewin (Gloucestershire Hospitals National Health Service [NHS] Foundation Trust), Norbert Blesing (Great Western Hospital), Dragana Milojkovic (Hammersmith Hospital), Timothy Moorby (King's Mill Hospital), Mamta Garg (Leicester Royal Infirmary), Fiona Dignan (Manchester Royal Infirmary), Deepak Mannari (Musgrove Park Hospital), Frances Wadelin (Nottingham City Hospital), Khalid Saja (Queen's Hospital), Sally Killick (Royal Bournemouth Hospital), Mallika Sekhar (University College Hospital), Richard Clark (Royal Liverpool University Hospital), Srinivas Pillai (Royal Stoke University Hospital), Josephine Crowe (Royal United Hospital), Rowena Thomas-Dewing (Salford Royal NHS Foundation Trust), Andrew Duncombe (Southampton General Hospital), Catherine Cargo (St James's University Hospital), Ciro Rinaldi (United Lincolnshire Hospitals NHS Trust), Zor Maung (Western General Hospital), John Laurie (Western Sussex NHS Foundation Trust), and Simon Watt (Wythenshawe Hospital). The support and time of participating patients and their families are gratefully acknowledged.
This trial is funded by Bloodwise under the Trials Acceleration Program. An unrestricted educational grant was provided to support the trial and adjunctive science by Novartis. Ruxolitinib was provided free of charge by Novartis. C.Y. was funded by grant C22436/A15958 from Cancer Research UK.
Authorship
Contribution: C.N.H., A.J.M., M.F.M., N.C.P.C., and C.Y. designed the MAJIC trial; C.N.H., S.F., C.Y., and A.H. wrote and reviewed the protocol; C.Y., A.P., A.H., and C.N.H. devised the statistical analysis plan; J.E., M.W., F.C., J.C., N.P., S.K., S. Ali, and S. Alimam recruited patients; A.P. conducted the statistical analysis, with statistical support from C.Y. and A.H.; A.C.D., R.S., and R.M. conducted the QoL analyses; C.N.H., A.J.M., A.P., C.Y., A.H., M.F.M., N.C.P.C., R.M., and A.C.D. interpreted the results; C.N.H., S.F., A.P., E.G., and C.Y. drafted the manuscript, and all authors reviewed the final version. C.N.H. is the guarantor.
Conflict-of-interest disclosure: C.N.H. has participated in advisory boards for Novartis, CTI, Baxaltra, and Celgene; was on speakers bureau for Novartis, CTI, Baxaltra, Shire, Gilead, INCYTE; received honoraria from Novartis, Shire CTI, Gilead, Baxaltra, INCYTE; and received research funding and travel, accommodation, and expenses from Novartis. A.J.M. has participated in advisory boards for Novartis, CTI, and Baxaltra; received honoraria from Novartis, Gilead, Shire, and Baxaltra; and also received research funding and travel, accommodation, and expenses from Novartis. F.C. and J.C. have received travel, accommodation, and expenses from Novartis. S.K. has participated in advisory boards for Novartis; received honoraria from Novartis, Shire, and Gilead; and received travel accommodation and expense from Novartis and Celgene. S. Ali received honoraria from Novartis and participated in advisory boards for Novartis. A.H. has participated in advisory boards for Novartis and was on the speakers bureau from Gilead. N.C.P.C. has participated in advisory boards for Novartis and received honoraria from Novartis and research support from Novartis. R.M. has consulted for Novartis, Ariad, and Galena and received research funding from Incyte, Gilead, CTI, NS Pharma, Celgene, and Promedior. M.F.M. has participated in advisory boards for Novartis and Gilead; received honoraria from Novartis, Shire, and Celgene; and received travel, accommodation, and expenses from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Claire N. Harrison, Department of Haematology, St Thomas' Hospital, Lambeth Palace Rd, London SE1 9RT, United Kingdom; e-mail: claire.harrison@gstt.nhs.uk.