Key Points
Human circulating leukocytes in humanized mice reproduce similar circadian oscillations as seen in humans.
A novel molecular clock network exhibiting opposite effects on regulating human and mouse leukocyte circadian rhythm is discovered.
Abstract
Many immune parameters show circadian rhythms during the 24-hour day in mammals. The most striking circadian oscillation is the number of circulating immune cells that display an opposite rhythm between humans and mice. The physiological roles and mechanisms of circadian variations in mouse leukocytes are well studied, whereas for humans they remain unclear because of the lack of a proper model. In this study, we found that consistent with their natural host species, mouse and human circulating leukocytes exhibited opposite circadian oscillations in humanized mice. This cyclic pattern of trafficking correlated well with the diurnal expression levels of C-X-C chemokine receptor 4, which were controlled by the intracellular hypoxia-inducible factor 1α/aryl hydrocarbon receptor nuclear translocator-like heterodimer. Furthermore, we also discovered that p38 mitogen-activated protein kinases/mitogen-activated 2 had opposite effects between mice and humans in generating intracellular reactive oxygen species, which subsequently regulated HIF-1α expression. In conclusion, we propose humanized mice as a robust model for human circadian studies and reveal insights on a novel molecular clock network in the human circadian rhythm.
Introduction
Several parameters of the immune system exhibit diurnal oscillations with a period of approximately 24 hours.1-3 These oscillations may help promote tissue recovery and the clearance of potentially harmful cellular elements from the circulation, and could be widely observed in oscillation of immune functions, innate immune response, and plasma levels of cytokines.4-6 In peripheral blood, the most striking circadian rhythm of the immune system is the change in numbers of circulating hematopoietic cells (lymphocytes, neutrophils, monocytes, and eosinophils).7-9 It is surprising that the numbers of most mature leukocytes and hematopoietic progenitor cells peak in the circulation during the night for humans and during the day for rodents, whereas the valley was usually seen during the day for humans and during the night for rodents.7,8 In rodent studies, it has been shown that the circadian rhythms of immune cell trafficking, adhesion, and migration are regulated by circadian control genes and chemokine systems such as C-X-C chemokine receptor 4 (CXCR4) and its ligand C-X-C motif chemokine 12 (CXCL12).7,9-13
Most mouse and human immune cells express circadian control genes and present a wide array of genes expressed with a 24-hour rhythm. At the core of the circadian machinery lies the aryl hydrocarbon receptor nuclear translocator-like (ARNTL1) protein, a basic helix–loop–helix (bHLH)-type transcription factor that forms homo- and heterodimeric complexes with several important proteins such as circadian locomotor output cycles kaputprotein (CLOCK) and bHLH family protein hypoxia-inducible factor 1α (HIF-1α).14-16 These dimers could control diverse developmental functions through transcriptional regulation by binding DNA at a consensus hexanucleotide sequence known as the enhancer box (E-Box).15,17,18 In mice, cCircadian oscillations of leukocyte numbers in both blood and tissues were completely abrogated in Arntl1 knockout animals in contrast to normal oscillations observed in heterozygous and wild-type littermates.7 HIFs are transcription factors that respond to decreases in available oxygen in the cellular environment and are able to regulate the expression of many molecules associated with immune cell function and migration.19 These findings provide valuable fundamental knowledge to associate the cellular environment with the inner circadian clock genes for the study of diurnal variants in circulating mouse immune cells, although the association between circadian control genes and the chemokine system is still not clearly explored. Early reports from human studies have confirmed many phenomena of the circadian rhythm of the human immune system, including the trafficking of circulating leukocytes8 ; however, the molecular basis underlying the circadian control of human immune cells still remains poorly understood because of the strict restriction of cohort recruitment, difficulty of human data collection, and lack of an in vivo human-specific platform for mechanism verification.
By transplanting human hematopoietic stem cells into immunodeficient mice, mice harboring a stable human immune system (humanized mice) have been developed.20,21 Humanized mice are a promising model for the study of many human diseases, hematopoiesis, and immune functions.21-23 As humanized mice are known to carry a chimerism of both mouse and human immune cells, here we tested a hypothesis that the circadian rhythms of the circulating human and mouse leukocytes in humanized mice followed similar patterns of both humans and mice in their natural host state, and explored the mechanisms that were important in controlling these processes. In this study, we show that circulating mouse and human leukocytes exhibited opposite circadian oscillations, and that these rhythmic changes were orchestrated by a novel molecular network involving phosphorylases, inner clock genes, cellular oxidative stress, and the chemokine system.
Methods
Mouse work
All manipulations with mice were approved by Agency for Science, Technology and Research (A*STAR) Institutional Animal Care and Use Committee. The diet provided was irradiated Teklad Global 18% Protein Rodent Diet (2918; Harlan Teklad, Madison, WI). Mice were housed in a sterile environment and only accessed under a BSL2 hood. Mice were fed, given water, and monitored daily for health, and cages were changed weekly.
NOD-SCID IL-2Rγ−/− (NSG) mice were purchased from The Jackson Laboratory and bred in a specific pathogen-free facility at the Biological Resource Centre in A*STAR. One- to 3-day-old NSG pups were irradiated with a 55-second exposure equaling 1.1 Gy and were transplanted with 1×105 CD34+ human fetal liver cells by intrahepatic injections. The mice were bled at 8 weeks posttransplantation to determine the fraction of human immune cell reconstitution. Reconstitution was calculated by (%hCD45+/[%hCD45+ + %mCD45+]). Mice reconstituted with 30% to 50% of human CD45+ cells were used for this study.
Age- and sex-matched NSG and humanized mice (8-12 weeks old) were housed to adapt to a cycle of a 12-hour-light/dark (lights on/off at 7 am/7 pm) at least 2 weeks before the start of experimentation. The room was maintained at 23 ± 2°C and at a constant humidity. All mice were housed in cages with filter tops and fed food ad libitum. For the antibody blocking experiment, 10-week-old humanized mice were treated with 75 µg anti-human/mouse CXCL12 antibody (MAB310; R&D) or anti-human CXCR4 antibody (MAB171; R&D) via tail vein injection 2 times a week, starting from 2 weeks before circadian analysis. For NAC experiment, 10-week-old humanized mice were treated with NAC (100 mg/kg/d; Sigma-Aldrich, St. Louis, MO) or phosphate-buffered saline (PBS) solution by subcutaneous administration for 2 weeks. For LY228820 treatment, 10-week-old humanized mice were given oral gavage treatment with LY2228820 (10 mg/kg, twice a day; Selleckchem, Houston, TX) for 2 weeks before circadian analysis. 5-FU (50 mg/mL) was obtained from ABIC (Petach-Tikra, Israel) and diluted to 10 mg/mL with PBS. Ten-week-old humanized mice were treated intraperitoneally with 10 mg 5-FU/k/day for 10 days. U0126 (Selleckchem) solution was prepared in DMSO as a stock solution of 10 μmol/L, and 25 µmol/kg/week was injected intraperitoneally into 10-week-old humanized mice for 2 weeks.
In the experimental chronic jet lag model, jet lag was induced in mice by 10 days of serial 8-hour advances of the light/dark cycle every 2 days.24 The control humanized mice were under light/dark 12:12, with the light on from 6 am to 6 pm. The circadian time (CT) corresponding to light onset (6 am) was defined as CT0, and offset (6 pm) as CT12.
Statistical analysis
Data were compared statistically, using the 1-sample, unpaired Student t test, or a 1-way analysis of variance. A post hoc Student Newman Keuls test was subsequently applied to determine which conditions were significantly different from each other, and a Tukey posttest was applied for multiple comparisons. Results were presented as means ± standard error, with values deemed statistically significant when P < .05.
Results
Mouse and human blood leukocytes showed distinct diurnal oscillations in humanized mice
Because of their immunodeficiency, mouse CD45+ (mCD45+) leukocytes in NSG mice consisted of only mouse Gr-1+ (mGr-1+) granulocytes and mouse F4/80+ (mF4/80+) monocytes/macrophages (supplemental Figure 1A, available on the Blood Web site), whereas in humanized mice, in addition to mCD45+ mouse leukocytes, there was a stable reconstitution of human CD45+ (hCD45+) leukocytes that included human CD3+ (hCD3+) T cells, human CD19+ (hCD19+) B cells, and other human hCD3−hCD19− (hCD3−hCD19−) cells (supplemental Figure 1B). We examined whether mouse and human leukocytes in the blood of NSG and humanized mice exhibited similar diurnal variations as in mouse and human. Blood samples were collected every 6 hours from 8-10-week-old NSG or humanized mice (from 7 am [Zeitgeber time, ZT0], on the onset of light). These mice were kept under a 12-hour light-dark cycle. Samples were analyzed by flow cytometry.
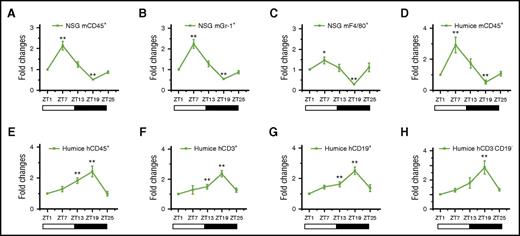
As shown in Figure 1, the total number of mouse blood leukocytes, mCD45+ cells (Figure 1A), their subsets, mGr-1+ granulocytes (Figure 1B), and mF4/80+ monocytes/macrophages (Figure 1C) varied in a diurnal manner; the peak was found 7 hours after the onset of light (ZT7), and the nadir was found 7 hours after lights off (ZT19). The same rhythm pattern of mCD45+ cells was also observed in humanized mice (Figure 1D). Interestingly, human blood leukocytes, hCD45+ cells (Figure 1E), the subsets of hCD3+ T cells (Figure 1F), hCD19+ B cells (Figure 1G), and hCD3−hCD19− cells (Figure 1H) produced significantly reversed circadian oscillations that had a peak at night (ZT19) and a trough in the daytime (ZT1). Together, these data demonstrated that mouse and human blood leukocytes in humanized mice could reproduce similar circadian rhythms with reversed diurnal variations similar to mice and humans. More intriguingly, it brought up a question of how mouse and human leukocytes populating the same in vivo environment were able to regulate circadian rhythm differently.
Circadian oscillations of circulating leukocytes in NSG and humanized mice. Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25) and analyzed for the number of leukocytes (n = 6). The cell counts of each point in individual mice were normalized to the initial cell count at 0 hours (ZT1). Shown are fold changes of cell counts of NSG mCD45+ cells (A), NSG mGr-1+ cells (B), NSG F4/80+ cells (C), humanized mice mCD45+ cells (D), humanized mice hCD45+ cells (E), humanized mice hCD3+ cells (F), humanized mice hCD19+ cells (G), and humanized mice hCD3−CD19− cells at different points against ZT1 (H). *P < .05; **P < .01.
Circadian oscillations of circulating leukocytes in NSG and humanized mice. Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25) and analyzed for the number of leukocytes (n = 6). The cell counts of each point in individual mice were normalized to the initial cell count at 0 hours (ZT1). Shown are fold changes of cell counts of NSG mCD45+ cells (A), NSG mGr-1+ cells (B), NSG F4/80+ cells (C), humanized mice mCD45+ cells (D), humanized mice hCD45+ cells (E), humanized mice hCD3+ cells (F), humanized mice hCD19+ cells (G), and humanized mice hCD3−CD19− cells at different points against ZT1 (H). *P < .05; **P < .01.
The diurnal oscillations of human and mouse blood leukocytes are both controlled by CXCL12/CXCR4-mediated rhythmic leukocyte trafficking
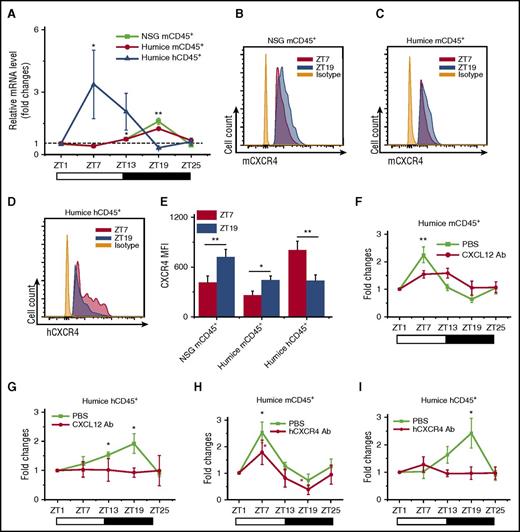
Recent mouse studies have demonstrated rhythmic trafficking of blood leukocytes into tissues.7 We compared the numbers of infiltrated human leukocytes in the organs by immunohistochemical staining for the pan-leukocyte marker hCD45. As shown in supplemental Figure 2, it appeared there were significantly more hCD45+ leukocytes found at ZT7 than ZT19 in mouse spleen, lung, liver, and kidney, which displayed a reciprocal diurnal rhythm to blood. This suggested that the diurnal oscillation of leukocytes could be a result of rhythmic leukocyte trafficking between peripheral blood and organs. We further examined the diurnal expression of CXCL12/CXCR4, a chemokine/chemokine receptor pair that has been shown to play important roles in rhythmic leukocyte trafficking in mice.7,9 To assay the expression of mouse and human CXCL12/CXCR4, blood and tissue samples were harvested at ZT7 and ZT19 points from NSG and humanized mice. Mouse CXCL12 (mCXCL12) was consistently detected, yet there was no obvious circadian rhythm of mCXCL12 in both protein expression levels in serum (supplemental Figure 3A) and mRNA expression levels in various organs (supplemental Figure 3B-H). Meanwhile, hCXCL12 mRNA was not detectable in any organ from humanized mice, which was not surprising, as these humanized mice primarily carried human immune cells and lacked CXCL12-producing human stromal cells (supplemental Figure 3I). Blood leukocytes were analyzed for the expression of CXCR4 by reverse transcription polymerase chain reaction (RT-PCR) and flow cytometry. Interestingly, CXCR4 showed an obvious day and night rhythm in both mRNA (Figure 2A) and surface protein levels (Figure 2B-E): At ZT7, mCXCR4 expression in mCD45+ cells from NSG and humanized mice was lower than at ZT19, whereas hCXCR4 expression in hCD45+ cells in humanized mice was higher at ZT19 than at ZT7. This expression pattern of CXCR4 correlated well with the diurnal oscillations of the numbers of blood leukocytes of the respective species.
CXCL12/CXCR4 controls the circadian rhythm of leukocyte trafficking. Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25). (A) Mouse and human CD45+ leukocytes were analyzed for CXCR4 expression by RT-PCR. Shown are fold changes of CXCR4 mRNA levels after normalizing data from each point in individual mice to the initial time ZT1 (n = 6). (B-E) Blood samples were analyzed by flow cytometry for the expression of CXCR4 at ZT7 and ZT19 (n = 6). Shown are representative flow cytometry plots and statistical analysis. (F-I) Humanized mice were treated with PBS and antibodies against CXCL12 (F-G) (n = 6) or CXCR4 (H-I) (n = 6), followed by sampling of blood from ZT1 to ZT25. The cell counts at each point in individual mice were normalized to the initial cell count at point ZT1. Shown are fold changes of numbers of mCD45+ cells and hCD45+ cells. *P < .05; **P < .01.
CXCL12/CXCR4 controls the circadian rhythm of leukocyte trafficking. Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25). (A) Mouse and human CD45+ leukocytes were analyzed for CXCR4 expression by RT-PCR. Shown are fold changes of CXCR4 mRNA levels after normalizing data from each point in individual mice to the initial time ZT1 (n = 6). (B-E) Blood samples were analyzed by flow cytometry for the expression of CXCR4 at ZT7 and ZT19 (n = 6). Shown are representative flow cytometry plots and statistical analysis. (F-I) Humanized mice were treated with PBS and antibodies against CXCL12 (F-G) (n = 6) or CXCR4 (H-I) (n = 6), followed by sampling of blood from ZT1 to ZT25. The cell counts at each point in individual mice were normalized to the initial cell count at point ZT1. Shown are fold changes of numbers of mCD45+ cells and hCD45+ cells. *P < .05; **P < .01.
Subsequently, in vivo blocking assays were carried out to further analyze the contribution of CXCL12/CXCR4 to the circadian rhythm of blood leukocytes. Blocking antibodies specifically recognizing CXCL12 (anti-mouse/human CXCL12) and human CXCR4 (anti-human CXCR4) were injected via tail vein into humanized mice to block the functions of CXCL12/CXCR4, followed by number counting of mouse and human leukocytes at different points. The results showed that the interference of CXCL12/CXCR4 interaction by blocking CXCL12 (Figure 2F-G) and CXCR4 (Figure 2H-I) significantly abrogated the circadian rhythm of mCD45+ cells and hCD45+ leukocytes. The analysis of blocking effects to the human hCD3+, hCD19+, and hCD3−CD19− subsets were also included (supplemental Figure 4). All these data indicated that CXCL12/CXCR4 not only was important for the circadian rhythm of mouse leukocytes, similar to reported wild-type mouse studies, but also was critical in mediating rhythmic trafficking of human leukocytes in the environment of humanized mice.
The circadian gene ARNTL1 is essential to the diurnal rhythm of human immune cells in humanized mice by regulating hCXCR4 expression
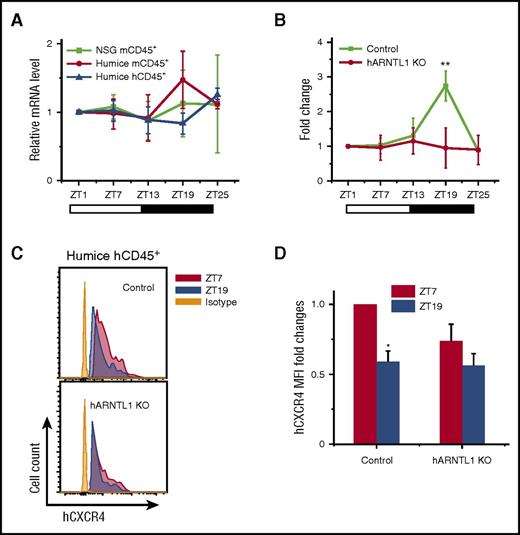
Given that the mouse and human leukocytes behaved differently in terms of circadian pattern in the same in vivo environment, such as the unitary expression of mCXCL12, we postulate that an internal mechanism exists within the immune cells to conserve their circadian rhythm. One possible mechanism is the internal transcriptional modulators that regulate the differential CXCR4 expression. CLOCK and ARNTL1 genes have been known to be essential components involved in the pathways critical to the generation of circadian rhythms.25,26 To assess the role of CLOCK, ARNTL1, and their associated genes in the circadian fluctuations of leukocytes, we analyzed the circadian expression of CLOCK and ARNTL1 and several CLOCK control genes in the blood leukocytes from NSG and humanized mice at different points. In mCD45+ leukocytes, a circadian rhythm was clearly detected for mouse circadian genes, such as period circadian protein homolog 1 (Per1) (supplemental Figure 5B), Per2 (supplemental Figure 5C), cryptochrome (Cry2) (supplemental Figure 5E), retinoic acid receptor-related orphan receptor α (Rora) (supplemental Figure 5G), E4 promoter-binding protein 4 (E4bp4) (supplemental Figure 5H), and d-site binding protein (Dbp) (supplemental Figure 5I). However, Clock (supplemental Figure 5A), Arntl1 (Figure 3A), Cry1 (supplemental Figure 5D), and the orphan nuclear receptor Rev-erba (supplemental Figure 5F) did not show an obvious circadian rhythm. In contrast to the expression of mouse circadian genes in mCD45+ cells, none of the above human circadian genes in hCD45+ cells exhibited obvious circadian oscillations (supplemental Figure 5 and Figure 3A).
hARNTL1 is critical in controlling circadian oscillations of circulating hCD45+leukocytes. (A) Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25) (n = 6). Mouse and human CD45+ leukocytes from different points were separated by cell sorting and analyzed for ARNTL1 expression by RT-PCR. Shown are fold changes of ARNTL1 mRNA levels after normalizing data from each point in individual mice to the initial point, ZT1. (B) Blood samples were taken from 12-week-old humanized mice (control) and hARNTL1−/− humanized mice (hARNTL1 KO) (n = 4). The cell counts of each time in individual mice were normalized to the initial cell numbers at time ZT1. Shown are fold changes of numbers of hCD45+ cells. (C) Representative flow cytometry plots of CXCR4 expression on hCD45+ cells from ZT7 and ZT19 points in control and hARNTL1 KO humanized mice. (D) Mean fluorescence intensity (MFI) quantifications of the expression of CXCR4 on hCD45+ cells from ZT7 and ZT19 (n = 4). Shown are the fold changes normalized to MFI of CXCR4 at ZT7 in control humanized mice. *P < .05; **P < .01.
hARNTL1 is critical in controlling circadian oscillations of circulating hCD45+leukocytes. (A) Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25) (n = 6). Mouse and human CD45+ leukocytes from different points were separated by cell sorting and analyzed for ARNTL1 expression by RT-PCR. Shown are fold changes of ARNTL1 mRNA levels after normalizing data from each point in individual mice to the initial point, ZT1. (B) Blood samples were taken from 12-week-old humanized mice (control) and hARNTL1−/− humanized mice (hARNTL1 KO) (n = 4). The cell counts of each time in individual mice were normalized to the initial cell numbers at time ZT1. Shown are fold changes of numbers of hCD45+ cells. (C) Representative flow cytometry plots of CXCR4 expression on hCD45+ cells from ZT7 and ZT19 points in control and hARNTL1 KO humanized mice. (D) Mean fluorescence intensity (MFI) quantifications of the expression of CXCR4 on hCD45+ cells from ZT7 and ZT19 (n = 4). Shown are the fold changes normalized to MFI of CXCR4 at ZT7 in control humanized mice. *P < .05; **P < .01.
Although the expression of ARNTL1 did not display a diurnal rhythm in both mouse and human cells in humanized mice, it is 1 of the most well-recognized key regulators for mouse and human circadian rhythm.13,25-27 To assess its function and validate our humanized mouse model, we performed clustered regularly interspaced short palindromic repeats (CRISPR)-Caspase 9 genetic editing to knock-out the human ARNTL1 (hARNTL1) gene in human CD34+ hematopoietic stem cells and to generate hARNTL1−/− hCD45+ cells in humanized mice (supplemental Figure 6A-B). The specificity and on-/off-target effects of ARNTL1 gene knockout in humanized mouse are shown in supplemental Figure 6C-E. Compared with the control group without gene editing, the circadian rhythm of human blood CD45+ leukocytes in hARNTL1−/− humanized mice was completely abolished (Figure 3B). Notably, the differential expression of hCXCR4 on hCD45+ cells at ZT7 and ZT19 was also abolished in hARNTL1−/− humanized mice (Figure 3C-D), which suggests a link between ARNTL1 and CXCR4. These results underscore the importance of hARNTL1 as an intracellular molecular clock required for maintaining rhythms of human blood leukocytes in humanized mice.
Opposite oscillations of cellular levels of reactive oxygen spices regulates the circadian expression of CXCR4 and leukocyte trafficking
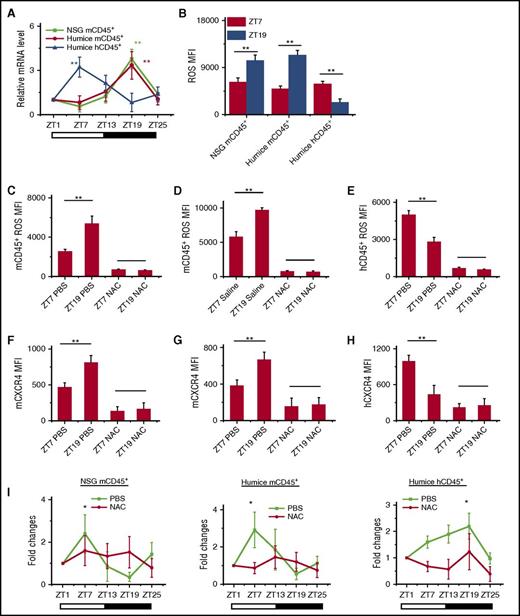
HIF-1α has been found to bind ARNTL1 and CLOCK to form a complex that regulates downstream CXCR4 expression.28-30 In addition, HIF-1α expression is known to have circadian rhythm in mice, although it is not yet studied in humans.29 Thus, we analyzed HIF-1α expressions in blood CD45+ leukocytes from NSG and humanized mice. The results showed that HIF-1α mRNA (Figure 4A) and protein (supplemental Figure 7A) had clear opposite circadian oscillations in both mouse and human CD45+ leukocytes, which matched well with the circadian rhythm of blood leukocytes and their CXCR4 expression. HIF-1α was reported to be regulated by intracellular levels of reactive oxygen spices (ROS).31 In addition, it had previously been suggested that oxidative stress might be linked to circadian rhythms, although the understanding of molecular mechanisms is still incomplete.32,33 Therefore, we examined whether ROS participated in regulating leukocyte trafficking via interactions with HIF-1α and CXCR4. As shown in supplemental Figure 7B and Figure 4B, ROS also produced circadian oscillations that were higher at ZT19 than at ZT7 in mCD45+ leukocytes in NSG and humanized mice, whereas it was the opposite in hCD45+ leukocytes, where ROS levels were higher at ZT7 and lower at ZT19. To further investigate the role of ROS in circadian rhythm of leukocytes, we treated NSG and humanized mice with N-acetylcysteine (NAC), which served as an inhibitor of ROS. In NSG and humanized mice treated with NAC, the ROS levels were significantly reduced in mouse and human leukocytes (supplemental Figure 7C and Figure 4C-E). Correspondingly, the circadian expression of CXCR4 (supplemental Figure 7D and Figure 4F-H) and HIF-1α mRNA (supplemental Figure 7E-F) on mCD45+ and hCD45+ leukocytes was also abrogated, which subsequently resulted in the loss of circadian rhythm of leukocyte trafficking (Figure 4I). In addition, fluorouracil (5-FU) is known to regulate ROS levels.34,35 We found that the injection of 5-FU into humanized mouse increased ROS levels in mouse and human CD45+ cells (supplemental Figure 8A-B), which consistently upregulated the expression of CXCR4 and HIF-1α on mCD45+ and hCD45+ leukocytes (supplemental Figure 8C-D). However, it also disrupted the circadian oscillation of CXCR4 and HIF-1α, which resulted in the abolishment of leukocyte circadian trafficking (supplemental Figure 8E-F). These data confirmed that the intracellular levels of ROS determined the circadian oscillations of leukocyte trafficking through the regulation of CXCR4.
Intracellular ROS regulates circadian oscillations of mouse and human leukocytes. (A) Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25) (n = 6). Mouse and human CD45+ leukocytes from different times were separated by cell sorting and analyzed for HIF-1α expression by RT-PCR. Shown are fold changes of HIF-1α mRNA levels after normalizing data from each point in individual mice to the initial time, ZT1. (B) Blood samples were taken from points ZT7 and ZT19 in 10-week-old NSG and humanized mice (n = 6). ROS expression levels in mCD45+ and hCD45+ cells were analyzed by flow cytometry. Shown is statistical analysis of ROS MFI. (C-H) Ten-week-old NSG and humanized mice were treated with PBS and NAC (n = 4). Blood samples were taken from points ZT7 and ZT19 and analyzed for ROS (C-E) and CXCR4 staining (F-H). (I) Blood samples were taken from 10-week-old humanized mice treated with PBS or NAC (n = 6). The number counts of mCD45+ and hCD45+ cells at each point in individual mice were normalized to the initial number account of time ZT1. Shown are fold changes of cell number. *P < .05; **P < .01.
Intracellular ROS regulates circadian oscillations of mouse and human leukocytes. (A) Blood samples were taken from 10-week-old NSG and humanized mice every 6 hours (ZT1-ZT25) (n = 6). Mouse and human CD45+ leukocytes from different times were separated by cell sorting and analyzed for HIF-1α expression by RT-PCR. Shown are fold changes of HIF-1α mRNA levels after normalizing data from each point in individual mice to the initial time, ZT1. (B) Blood samples were taken from points ZT7 and ZT19 in 10-week-old NSG and humanized mice (n = 6). ROS expression levels in mCD45+ and hCD45+ cells were analyzed by flow cytometry. Shown is statistical analysis of ROS MFI. (C-H) Ten-week-old NSG and humanized mice were treated with PBS and NAC (n = 4). Blood samples were taken from points ZT7 and ZT19 and analyzed for ROS (C-E) and CXCR4 staining (F-H). (I) Blood samples were taken from 10-week-old humanized mice treated with PBS or NAC (n = 6). The number counts of mCD45+ and hCD45+ cells at each point in individual mice were normalized to the initial number account of time ZT1. Shown are fold changes of cell number. *P < .05; **P < .01.
The p38 mitogen-activated protein kinases/mitogen-activated 2 pathway regulated opposite circadian rhythms of mouse and human leukocytes via ROS
It has been suggested that the p38 mitogen-activated protein kinases/mitogen-activated 2 (p38MAPK/MK2) signaling pathway exhibits opposite effects on the production of ROS between mice and humans at cellular levels. In human cancer cells, the phosphorylation of p38MAPK/MK2 stimulates the production of ROS,36,37 whereas in mouse cells, the same phosphorylation results in reduced ROS production.37 This mechanism might help explain the differential levels of ROS in mouse and human leukocytes in our model.
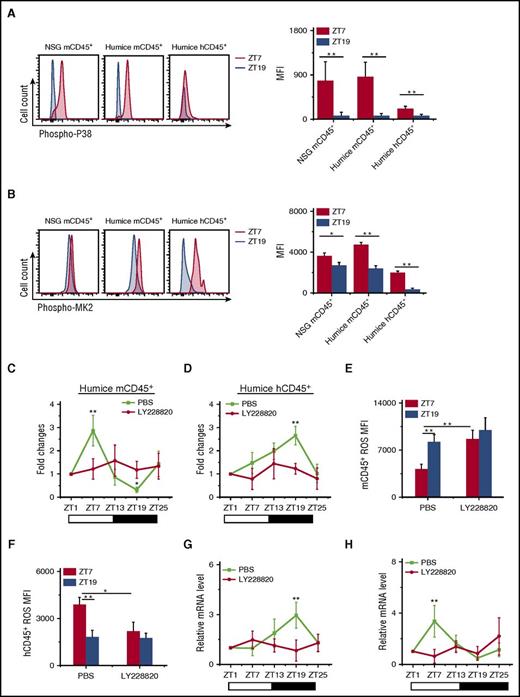
To investigate whether the p38MAPK/MK2 pathway was involved in circadian rhythm of leukocyte trafficking, we measured the phosphorylation of p38MAPK and MK2 at ZT7 and ZT19. Very clearly, both p38MAPK and MK2 had much higher phosphorylation levels at ZT7 compared with ZT19 (Figure 5A-B), which corresponded to the upregulation and downregulation of ROS at ZT7 in human and mouse leukocytes, respectively (Figure 4B).
Opposite function of p38MAPK/MK2 on ROS generation and circadian oscillations of circulating mouse and human leukocyte. (A-B) Blood samples were taken from points ZT7 and ZT19 in 10-week-old NSG and humanized mice (n = 6). Shown are representative stain and statistical analysis of phosphorylated p38MAPK (phospho-P38) (A) and phosphorylated MK2 (phospho-MK2) (B). (C-H) Blood samples were taken from 10-week-old humanized mice treated with PBS or LY228820 (n = 6). The number counts of mCD45+ (C) and hCD45+ (D) cells at each point in individual mice were normalized to the initial number at time ZT1. Shown are fold changes of cell number. The ROS MFI in mCD45+ (E) and hCD45+ (F) cells at points ZT7 and ZT19 were analyzed. The 24-h circadian oscillations of HIF-1α mRNA in mCD45+ (G) and hCD45+ (H) cells are shown. *P < .05; **P < .01.
Opposite function of p38MAPK/MK2 on ROS generation and circadian oscillations of circulating mouse and human leukocyte. (A-B) Blood samples were taken from points ZT7 and ZT19 in 10-week-old NSG and humanized mice (n = 6). Shown are representative stain and statistical analysis of phosphorylated p38MAPK (phospho-P38) (A) and phosphorylated MK2 (phospho-MK2) (B). (C-H) Blood samples were taken from 10-week-old humanized mice treated with PBS or LY228820 (n = 6). The number counts of mCD45+ (C) and hCD45+ (D) cells at each point in individual mice were normalized to the initial number at time ZT1. Shown are fold changes of cell number. The ROS MFI in mCD45+ (E) and hCD45+ (F) cells at points ZT7 and ZT19 were analyzed. The 24-h circadian oscillations of HIF-1α mRNA in mCD45+ (G) and hCD45+ (H) cells are shown. *P < .05; **P < .01.
To further investigate the role of p38MAPK and MK2, a trisubstituted imidazole derivative, LY228820, has been used to specifically inhibit MK2 phosphorylation.38 Injection of LY228820 into humanized mice resulted in the abolishment of the circadian rhythm in both mouse and human CD45+ leukocytes (Figure 5C-D). LY228820 also disrupted the circadian oscillation of ROS (Figure 5E-F) and HIF-1α (Figure 5G-H) in both mCD45+ and hCD45+. Interestingly, the inhibition of the MK2 phosphorylation by LY228820 increased ROS level in mCD45+ (Figure 5E) and decreased ROS level in hCD45+ (Figure 5F) at ZT7, which confirmed the connection between p38MAPK/MK2 and ROS. In contrast, the blocking of ROS by NAC did not affect the phosphorylation of p38 (supplemental Figure 9A-B) and MK2 (supplemental Figure 9C-D), which revealed that p38MAPK/MK2 is the upstream of ROS.
Extracellular signal-regulated kinases (ERK) and p38 MAPK-activated protein kinases are the same family of protein kinases, but with diverse biological functions.39 We also analyzed the potential role of ERK1/2 in this model. The inhibition of ERK1/2 phosphorylation by its specific inhibitor U0126 did not change the circadian fluctuations of leukocytes (supplemental Figure 10A-B). All these data illustrate that the p38MAPK/MK2 pathway is the critical upstream component for the development of the circadian rhythm in leukocyte trafficking.
Jet lag impaired the circadian rhythm of blood leukocyte in humanized mice
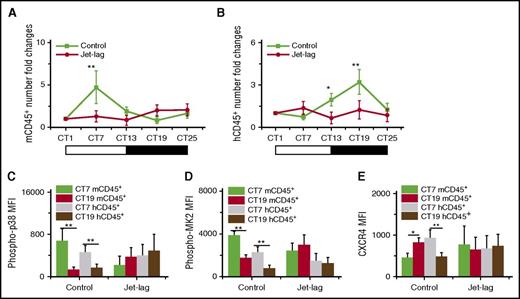
Jet lag induces physiology stress, including oxygen stress. It has been proven that phase shift induced by experimental chronic jet lag could impair the oscillations of mice blood leukocytes, and the signal of p38/MK2 could be activated in response to stress-related stimuli.7,40 To investigate whether jet lag could influence the circadian rhythm of leukocyte trafficking in humanized mouse, we applied an experimental chronic jet lag model to humanized mouse.24 After the induction of jet lag, the jet lag group clearly lost the circadian rhythm of both mouse and human blood leukocytes (Figure 6A-B). Furthermore, the oscillation of p38/MK2 phosphorylation (Figure 6C-D) and CXCR4 expression (Figure 6E) in the jet lag group was also disrupted compared with in the control group. Because this jet lag model is linked to light-phase changes, these results suggested that the environmental conditions, likely the light phase, are the outer control of the circadian rhythm of leukocytes.
The effect of experimental chronic jet lag on the circadian rhythm of mouse and human leukocytes. Blood samples were taken from 10-week-old humanized mice without (Control) or with (Jet-lag) the induction of jet lag at different times (n = 6). The CT corresponding to light onset (6 am) was defined as CT0. The number counts of mCD45+ (A) and hCD45+ (B) cells at each time in individual mice were normalized to the initial number at time CT1. Shown are fold changes of cell number. The MFI levels of phosphorylation of p38 (C) and MK2 (D) in mCD45+ and hCD45+ cells at CT7 and CT19. (E) The MFI levels of CXCR4 in mCD45+ and hCD45+ cells at CT7 and CT19. *P < .05; **P < .01.
The effect of experimental chronic jet lag on the circadian rhythm of mouse and human leukocytes. Blood samples were taken from 10-week-old humanized mice without (Control) or with (Jet-lag) the induction of jet lag at different times (n = 6). The CT corresponding to light onset (6 am) was defined as CT0. The number counts of mCD45+ (A) and hCD45+ (B) cells at each time in individual mice were normalized to the initial number at time CT1. Shown are fold changes of cell number. The MFI levels of phosphorylation of p38 (C) and MK2 (D) in mCD45+ and hCD45+ cells at CT7 and CT19. (E) The MFI levels of CXCR4 in mCD45+ and hCD45+ cells at CT7 and CT19. *P < .05; **P < .01.
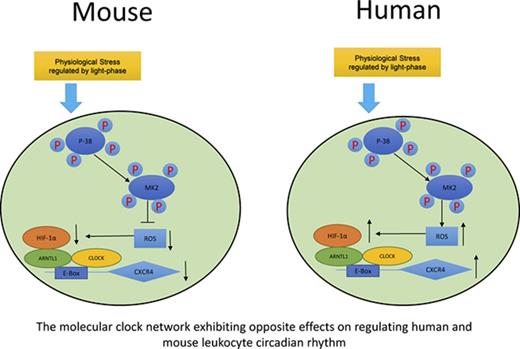
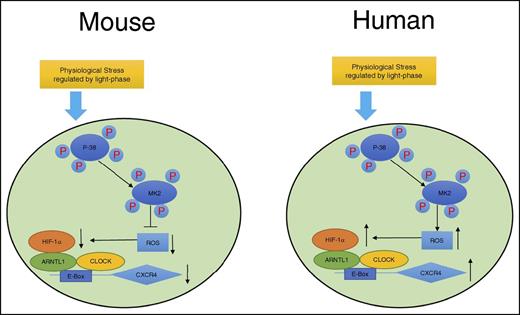
In summary, as shown in Figure 7, we propose a model demonstrating that although exposed to the same environment such as light-phase changes, mouse and human circulating leukocytes exhibit opposite circadian oscillations of trafficking. A novel pathway is proposed by our work that this differential phenomenon between mouse and human is associated with the opposite effects of p38MAPK/MK2 in the generation of intracellular ROS, which subsequently regulates the production of HIF-1α. HIF-1α and ARNTL1, together with CLOCK, form a complex, bind to the upstream element E-Box of the CXCR4 gene, and regulate the transcriptional activities of CXCR4, which controls the trafficking of leukocytes.
Sketch map of molecular control of circadian rhythm in mouse and human leukocytes. Mouse and human leukocytes can be stimulated by the physiological stress related to light phase during the day time. Subsequently, p38MAPK/MK2 is activated by phosphorylation. The downstream ROS levels respond differentially to p38MAPK/MK2 activation because of the possible opposite functions of MK2 between mouse and human. ROS positively correlates to the binding capacity of HIF-1α/ARNTL1/CLOCK complex on the promoter of CXCR4, which regulates the expression of CXCR4 and results in the opposite circadian oscillation between mouse and human leukocytes.
Sketch map of molecular control of circadian rhythm in mouse and human leukocytes. Mouse and human leukocytes can be stimulated by the physiological stress related to light phase during the day time. Subsequently, p38MAPK/MK2 is activated by phosphorylation. The downstream ROS levels respond differentially to p38MAPK/MK2 activation because of the possible opposite functions of MK2 between mouse and human. ROS positively correlates to the binding capacity of HIF-1α/ARNTL1/CLOCK complex on the promoter of CXCR4, which regulates the expression of CXCR4 and results in the opposite circadian oscillation between mouse and human leukocytes.
Discussion
It has been well recognized that the body clock can significantly affect many biological processes in humans, including the efficacy and adverse effects of drug treatments.41-43 Thus, increasing effort has been spent to study the phenomena of circadian rhythm of leukocyte trafficking and other immune functions. Several investigations have demonstrated that the number of circulating leukocytes displays diurnal oscillation in humans and rodents; the peak occurs at night in humans and during the day in rodents.7,8 In rodents, it has been suggested that these oscillations are regulated by circadian hematopoietic cell recruitment between peripheral blood and tissues such as the bone marrow via CXCL12/CXCR4.7,9 Another study proved that circadian oscillations of leukocytes were completely disrupted in mice deficient in Arntl1.7 However, little is known about humans because of the lack of human-specific study platforms.
In this new model, humanized mice carry both mouse and human immune cells and are able to retain their own species-specific nature, even though they are growing in the same environment; mouse circulating leukocytes show the same circadian oscillation as the wild-type mice, peak at ZT7 and nadir at ZT19, whereas human circulating leukocytes display opposite oscillations, peak at ZT19 and nadir at ZT7. In our humanized mice, only mCXCL12 is detectable because of the lack of human stromal cells producing hCXCL12. In addition, mCXCL12 is expressed at consistent levels without obvious circadian changes. Hence, in this identical in vivo environment in humanized mice, our study proves that intracellular regulation mechanisms within mouse and human leukocytes, rather than extracellular factors, play more critical roles in determining how to traffic among different locations and generate circadian rhythm. In other words, this model helps explain that the key regulating mechanisms of circadian rhythms have been programmed inside the leukocytes of different species themselves, despite the environment in which they are placed.
We further demonstrate that the expression of mCXCR4 and hCXCR4 is oppositely regulated after circadian rhythm in circulating mouse and human leukocytes. This led us to search for genes regulating CXCR4 expression. A complex of ARTNL1, CLOCK, and HIF-1α is well known to be the key regulator of CXCR4.27,44-48 Our evidence showed that the hARTNL1 knockout arrested the circadian expression of CXCR4 and abrogated the circadian oscillations of human leukocytes. Because ARTNL1 does not have circadian expression, it is probably does not serve as a factor that differentiates between mouse and human cell trafficking, as for CLOCK. Thus, we considered HIF-1α, the expression of which in mouse and human leukocytes followed the same circadian rhythms as these 2 species. HIF-1α further linked us to ROS, as it is reported to positively regulate HIF-1α expression, and its intracellular levels are also positively related to the expression of CXCR4 and trafficking of mouse and human leukocytes. Inhibition of ROS production disrupted the circadian oscillation of CXCR4 expression, and hence the leukocyte trafficking. Interestingly, we found that the upstream activated/phosphorylated p38MAPK/MK2 have differential effects in ROS induction in mouse and human cells. Furthermore, the phosphorylation of p38MAPK/MK2 is also proven to have circadian rhythms and regulate circadian leukocyte trafficking. On the basis of the results from this humanized mouse model, we discovered a unique pathway of p38MAPK/MK2-ROS-HIF-1α-ARNTL1-CXCR4.
In conclusion, we have developed the first in vivo model that successfully reproduces a human-specific circadian rhythm of leukocyte trafficking, and potentially other biological functions. With this novel model, study of the environmental and molecular parameters that are involved in the biological processes of the circadian rhythm, especially the human-specific mechanisms, becomes possible. More important, this study will likely offer useful tools and open new insights for human healthcare and research, as the advancement of our knowledge on circadian rhythms to timed medical treatment will potentially yield better outcomes in many human diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Joint Council Office Development Programme 1334k00082, A*STAR, Singapore (Q.C.), and by the Eradication of Hepatitis B Virus Translational and Clinical Research (HBV TCR) Program (NMRC/TCR/014-NUHS/2015), Singapore National Medical Research Council. Q.C. is also supported by the Singapore National Research Foundation Fellowship (NRF-NRFF2017-03).
Authorship
Contribution: Y.Z. designed and performed experiments, analyzed and interpreted data, and prepared the manuscript; M.L., X.Y.C., and S.Y.T. performed experiments; S.S. prepared the manuscript; Y.F., E.L., K.T.E.C., and T.C.T. contributed the research tools and reagents and prepared the manuscript; and Q.C. designed experiments, conceived the study, supervised the project and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qingfeng Chen, Institute of Molecular and Cell Biology, Proteos, 61 Biopolis Dr, Singapore 138673; e-mail: qchen@imcb.a-star.edu.sg.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal