Key Points
WT1-specific TCR-redirected T-cell therapy for AML and MDS is safe, and the T cells persisted in vivo and trafficked to bone marrow.
Transient decreases of leukemic cell in bone marrow were shown.
Abstract
Wilms’ tumor 1 (WT1) is constantly expressed in leukemic cells of acute leukemia and myelodysplastic syndrome (MDS). A T-cell receptor (TCR) that specifically reacts with WT1 peptide in the context of HLA-A*24:02 has been identified. We conducted a first-in-human trial of TCR–gene transduced T-cell (TCR–T-cell) transfer in patients with refractory acute myeloblastic leukemia (AML) and high-risk MDS to investigate the safety and cell kinetics of the T cells. The WT1-specific TCR-gene was transduced to T cells using a retroviral vector encoding small interfering RNAs for endogenous TCR genes. The T cells were transferred twice with a 4-week interval in a dose-escalating design. After the second transfer, sequential WT1 peptide vaccines were given. Eight patients, divided into 2 dose cohorts, received cell transfer. No adverse events of normal tissue were seen. The TCR-T cells were detected in peripheral blood for 8 weeks at levels proportional to the dose administered, and in 5 patients, they persisted throughout the study period. The persisting cells maintained ex vivo peptide-specific immune reactivity. Two patients showed transient decreases in blast counts in bone marrow, which was associated with recovery of hematopoiesis. Four of 5 patients who had persistent T cells at the end of the study survived more than 12 months. These results suggest WT1-specific TCR-T cells manipulated by ex vivo culture of polyclonal peripheral lymphocytes survived in vivo and retained the capacity to mount an immune reaction to WT1. This trial was registered at www.umin.ac.jp as #UMIN000011519.
Introduction
Despite establishing standard chemotherapies and supportive care for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), less than half of patients with AML are cured, and the average survival of patients with MDS is only 2.5 years after diagnosis.1 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is only a curative therapy for MDS and refractory AML. The therapeutic rationale for allo-HSCT is thought to be the graft-vs-tumor effect. Graft-vs-tumor is mediated by part of a donor’s immune system that recognizes specific antigens expressed in/on tumor cells. Donor immune systems also recognize allo-antigens expressed on recipient normal cells and cause graft-versus-host disease, one of the major obstacles to successful allo-HSCT. These allogeneic immune reactions are often associated with serious adverse events. Bcause patients with MDS and AML, especially elderly individuals, do not always benefit from allo-HSCT, a new type of immune-mediated therapy is desired.
Tumor-specific T-cell receptor (TCR)-gene transduced T cells (TCR-T cells) and chimeric antigen receptor-gene transduced T cells (CAR-T cells) have been developed as effective immune cell therapies for refractory tumors.2,3 TCR-T cells recognize peptide antigens that are presented by human leukocyte antigen (HLA) molecules. CAR-T cells recognize cell surface antigens with a single-chain variable fragment portion of the CAR. Antigens that are specifically expressed in tumor cells, such as cancer-testis antigens, and mutated proteins are attractive targets of immunotherapy. Antigens that are abundantly expressed in tumor cells are also potential targets. Thus, discovery of ideal target antigens and selection of immune receptors with appropriate receptor-antigen affinity is important for establishment of safe immune cell therapy.
Wilms’ tumor 1 (WT1) is expressed in a majority of MDS and AML cells, and messenger RNA (mRNA) of WT1 in peripheral blood is monitored as a marker of minimal residual disease in AML and MDS.4 Several WT1 protein-derived epitopes that are recognized by cytotoxic T lymphocytes (CTLs) along with HLA have been determined.5-7 In vitro studies and WT1 peptide vaccine clinical trials have demonstrated that WT1-specfic CD8+ T cells with cytotoxic activity can be induced.5-7
A CTL clone, TAK-1, which recognizes WT1235-243 peptide in an HLA-A*24:02-restricted manner was previously established.5 We constructed a retrovirus vector, pMS3-WT1-siTCR, for transduction of T cells with TCR-α and TCR-β chains derived from TAK-1. Retroviral transduction of WT1-specific TCR genes confers WT1 specificity on 45.1% to 67.2% of CD8+ T cells. These T cells exhibited HLA-A*24:02-restricted cytotoxicity against WT1-expressing tumor cells.8
In addition to the recognition of antigens expressed in normal tissues, antigen-specific TCR-gene transfer may cause serious autoimmune disease by mispairing of introduced and endogenous TCR chains that recognize autoantigens.9,10 Mispairing of TCR chains may also cause reduced antitumor responses. We established a retroviral vector system encoding small interfering RNAs (siRNAs) for endogenous TCR genes to eliminate TCR-mispairing.11
Here, we present a first-in-human trial of adoptive transfer of WT1-specfic TCR-T cells that were prepared with a siRNA-encoding viral vector for patients with refractory AML and high-risk MDS.
Methods
Preparation of WT1-specific TCR-T cells
Lymphocytes were isolated from 100 or 200 mL of heparinized peripheral blood of each patient by density centrifugation. In a cell-processing facility, the lymphocytes were cultured with interleukin 2, anti-CD3 antibody, and RetroNectin (Takara Bio Inc.). Proliferating lymphocytes were infected with the retroviral vector, pMS3-WT1-siTCR, which was constructed from DNA encoding WT1235-243/HLA-A*24:02-specific TCR-α and TCR-β chains.8 It also contains interfering RNA constructs that specifically downregulate endogenous TCR.11 After 10 to 14 days in culture, the lymphocytes were harvested and then frozen until use. The cells were tested for qualification before transfer.
Interferon (IFN)-γ producing cells were assayed as described previously,12 with some modifications. Briefly, the cells were stimulated with WT1 peptide. Brefeldin A was added, and they were then incubated with anti-CD8 monoclonal antibody (Becton Dickinson). The cells were stained intracellularly with anti-IFN-γ monoclonal antibody.
Study design
This study was a phase 1, cell dose-escalating clinical trial of WT1-specific TCR-T cell transfer for treatment of patients with refractory AML and high-risk MDS expressing WT1 antigen. The primary objective was to determine clinical safety, and the secondary objectives were to assess cell kinetics of the WT1-specific TCR-T cells in peripheral blood and to measure infiltration into the bone marrow. The other aims were to determine WT1-specific immune responses and clinical responses.
Patients were eligible for inclusion in the study if they met each of the following criteria: had refractory AML or high-risk MDS with WT1 mRNA expression; were positive for HLA-A*24:02; had a performance status of 0, 1, or 2; were aged 20 years or older; had a life expectancy of 4 months or more, and did not have impaired organ function. WT1 mRNA expression levels were determined using a kit from the Diagnostic Division of Otsuka Pharmaceutical Co., Ltd. (Tokushima, Japan). Peripheral blood samples expressing 200 or more polymerase chain reaction (PCR)-amplified copies were judged as WT1 positive.
The patients were divided into 3 cohorts of 3 patients each: cohort 1, 2 × 108 cells (whole cells including TCR-T cells) per dose; cohort 2, 1 × 109 cells per dose; cohort 3, 5 × 109 cells per dose. In cases of manufactured cell products that did not reach the target doses, patients were allowed to receive cell transfer treatment as extracohort cases, if they were willing. Clinical safety was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.02. Clinical responses were assessed according to the criteria proposed by Cheson et al.13,14 All safety information was collected and evaluated, and dose escalation was judged by the Independent Data and Safety Committee.
The study was conducted in accordance with the current version of the Declaration of Helsinki. Written informed consent was obtained from all patients participating in this study. The protocol was approved by the institutional review board of Mie University Hospital and affiliated institutions. It was also approved by the Ministry of Health, Labor, and Welfare of Japan. This clinical trial was registered with the UMIN Clinical Trials Registry (UMIN000011519).
Treatment protocol
After preparation of WT1-specific TCR-T cells, patients were administered the cells intravenously twice on days 0 and 28. On days 30 and 44, patients were subcutaneously administered 300 μg mutated WT1235-243 peptide (CYTWNQMNL; PolyPeptide Laboratories)7 emulsified with incomplete Freund’s adjuvant (Montanide ISA-51VG; SEPPIC). On day 58, safety and clinical responses were assessed.
Cell kinetics and bone marrow trafficking of WT1-specific TCR-T cells
Heparinized peripheral blood was collected at baseline and at predetermined times during the 58-day period. On days 28 and 58, bone marrow samples were aspirated, if possible. Peripheral blood mononuclear cells (PBMCs) and bone marrow samples were isolated and cryopreserved. DNA samples were extracted from cryopreserved PBMCs or bone marrows. Transduced gene copy number was measured by quantitative PCR (qPCR), using the Cycleave PCR Core Kit (Product code CY501, Takara Bio Inc.) and specific primers (Provirus Copy Number Detection Primer Set, Human (Product code 6167, Takara Bio Inc.)), according to the manufacturer’s instructions. The copy number of the WT1-specific TCR DNA in the patient’s PBMCs is presented as the number of DNA copies per 105 PBMCs (A × 103). The vector copy number in TCR-T cells was also measured by the same qPCR assay, and is indicated as the number of DNA copies per cell (B). The percentage of cells that bear the retroviral DNA in PBMCs was obtained by dividing (A) by (B) (supplemental Tables 2 and 3, available on the Blood Web site).
Tetramer analysis and enzyme-linked immunospot assay
CD8+ T cells were isolated from patients’ PBMCs collected at each time point, and were then cultured with the WT1 peptide−pulsed non-CD8+ T cells. The cells were cultured in the presence of interleukin 2, interleukin 7, and 10% human AB serum. After 8 days in culture, the stimulated CD8+ T cells were used as effector cells in tetramer analysis and in an enzyme-linked immunospot (ELISPOT) assay, as described previously.15
Using WT1 peptide/HLA-A*24:02 tetramer, the frequencies of WT1 TCR-positive T cells were analyzed by flow cytometry. In parallel, ELISPOT assay was performed by targeting T2A24 cells pulsed with WT1 peptide.
The ELISPOT assay was performed as described previously with some modifications.16 Briefly, an ELISPOT plate (MAHA S4510, Millipore) was coated with anti-human IFN-γ monoclonal antibody (R&D Systems). A total of 5 × 104 sensitized CD8+ T cells and 1 × 105 peptide-pulsed T2A24 cells, or nonpulsed T2A24 cells, was placed in each well of the ELISPOT plate. After incubation, the plate was washed and then supplemented with biotinylated capture antibody and incubated overnight. After reacting with streptavidin-alkaline phosphatase conjugate, the plate was stained with an alkaline phosphatase conjugate substrate kit (Bio-Rad). Spots were counted using an ELISPOT Plate Reader (ImmunoSpot, CTL-Europe GmbH).
Results
Preparation of WT1-specific TCR-T cells
Ex vivo proliferation of the WT1-specific TCR-T cells was variable among the individuals (supplemental Figure 1). Three patients, TCR-WT-02-M01, TCR-WT-03-M02, and TCR-WT-06-E03, who were allocated to cohort 1, reached cell numbers of 2 × 108 cells per dose. Five patients allocated to cohort 2 achieved 1 × 109 cell dose per infusion, of whom 3 patients reached the planned cell numbers and the other 2 patients, TCR-WT-09-N05 and TCR-WT-10-F02, did not reach the target numbers (Table 1). As for the cohort 3, it was unlikely that the planned number of TCR-T cells could be prepared, so we decided not to register any cases in cohort 3 and complete the study. After preparing WT1-specific TCR-T cells, we analyzed their cell-surface phenotypes (supplemental Table 1). More than 99% were CD3+ T cells. Seven patients had CD8+ T cell dominance, and 1 patient had equal distributions of CD4+ and CD8+ T cells. Among the CD8+ T-cells, 5.0% to 52.5% of lymphocytes responded to the WT1 peptide, approximating the percentage of T cells transduced with the WT1-specific TCR gene. WT1 tetramer-positive CD8+ T cells ranged from 1.3% to 33.8% (supplemental Table 1).
Characteristics of patients who received WT1-TCR-gene transduced lymphocyte transfer
| Cohort, patient ID . | Age (y) . | Sex . | Number of T cells infused, per dose (×108) . | Disease* . | Karyotype . | Prior therapy . | Disease characteristics before TCR-T cell transfer . |
|---|---|---|---|---|---|---|---|
| 1 | |||||||
| TCR-WT-02-M01 | 68 | Male | 2 | MDS, RCMD | 46, XY | None | Erythroblastosis, blood transfusion dependency |
| TCR-WT-03-M02 | 65 | Female | 2 | AML/MRC | Multiple abnormalities | Cytarabine/aclarubicin/G-CSF | 30% blasts in BM |
| TCR-WT-06-E03 | 69 | Male | 2 | MDS, RAEB-1 | 46, XY | None | AML/MRC, 24.8% blasts in BM |
| 2 | |||||||
| TCR-WT-07-M03 | 71 | Male | 10 | MDS, REAB | 46, XY | Azacitidine | 10% blasts in BM |
| TCR-WT-08-N04 | 70 | Male | 10 | MDS, REAB-2 | Multiple abnormalities | Azacitidine | AML/MRC, 40.5% blast in BM |
| Extracohort | |||||||
| TCR-WT-09-N05† | 76 | Male | 9.7 | AML/MRC | 46,XY, t(4;21)(q21;q22), add (7)(q22), add(14)(q24) | Azacitidine, cytarabine/aclarubicin/G-CSF | 87% blasts in BM |
| 5.6 | |||||||
| TCR-WT-10-F02 | 68 | Male | 6.5 | MDS, RCMD | 46, XY, del(20)(q1?) | None | Blood transfusion dependency |
| 2 | |||||||
| TCR-WT-11-E04 | 64 | Male | 10 | MDS, RAEB-1 | 46, XY | None | AML/MRC, 24.8% blasts in BM |
| Cohort, patient ID . | Age (y) . | Sex . | Number of T cells infused, per dose (×108) . | Disease* . | Karyotype . | Prior therapy . | Disease characteristics before TCR-T cell transfer . |
|---|---|---|---|---|---|---|---|
| 1 | |||||||
| TCR-WT-02-M01 | 68 | Male | 2 | MDS, RCMD | 46, XY | None | Erythroblastosis, blood transfusion dependency |
| TCR-WT-03-M02 | 65 | Female | 2 | AML/MRC | Multiple abnormalities | Cytarabine/aclarubicin/G-CSF | 30% blasts in BM |
| TCR-WT-06-E03 | 69 | Male | 2 | MDS, RAEB-1 | 46, XY | None | AML/MRC, 24.8% blasts in BM |
| 2 | |||||||
| TCR-WT-07-M03 | 71 | Male | 10 | MDS, REAB | 46, XY | Azacitidine | 10% blasts in BM |
| TCR-WT-08-N04 | 70 | Male | 10 | MDS, REAB-2 | Multiple abnormalities | Azacitidine | AML/MRC, 40.5% blast in BM |
| Extracohort | |||||||
| TCR-WT-09-N05† | 76 | Male | 9.7 | AML/MRC | 46,XY, t(4;21)(q21;q22), add (7)(q22), add(14)(q24) | Azacitidine, cytarabine/aclarubicin/G-CSF | 87% blasts in BM |
| 5.6 | |||||||
| TCR-WT-10-F02 | 68 | Male | 6.5 | MDS, RCMD | 46, XY, del(20)(q1?) | None | Blood transfusion dependency |
| 2 | |||||||
| TCR-WT-11-E04 | 64 | Male | 10 | MDS, RAEB-1 | 46, XY | None | AML/MRC, 24.8% blasts in BM |
MDS subtypes were classified by World Health Organization (WHO) criteria.
BM, bone marrow; G-CSF, granulocyte colony-stimulating factor; MRC, myelodysplasia-related changes; RCMD, refractory cytopenia with multilineage dysplasia; REAB, refractory anemia with excess of blasts.
At clinical trial entry, classified by WHO criteria.
TCR-gene transduced T cells for the first and the second infusion were separately prepared.
Patients receiving transfer of WT1-specific TCR-T cells
From October 2013 to June 2016, 12 patients were enrolled in the clinical trial. From the patients, WT1-specific TCR-T cells were prepared. Four patients were withdrawn before lymphocyte transfer because their general condition deteriorated as a result of rapid disease progression. The remaining 8 patients were treated with the TCR-T cells at 1 of 2 doses: 2 × 108 cells/dose (cohort 1, 3 patients), or 1 × 109 cells/dose (cohort 2, 5 patients). Two patients in cohort 2, TCR-WT-09-N05 and TCR-WT-10-F02, did not meet the target T cell number; instead, they received the actual cell doses that were prepared: 9.7 × 108 and 5.6 × 108 cell doses in TCR-WT-09-N05, and 6.5 × 108 cells in TCR-WT-10-F02, respectively (Table 1).
As shown in Table 1, 2 patients had AML transformed from MDS, and the remaining 6 patients had MDS. In these 6 patients with MDS, 3 developed transformation to AML. Four patients received prior therapies of azacitidine or low-dose chemotherapy combined with cytosine arabinoside and aclarubicin.
WT1 mRNA levels in peripheral blood at study entry ranged from 340 to 69 000 copies (supplemental Figure 2).
Safety and adverse events
None of the 8 patients experienced any adverse events greater than scale 3 after T-cell transfer. No dose-limiting toxicities were seen. In 7 patients, we observed skin reactions such as redness and induration, graded as 1, at the peptide vaccine sites (Table 2).
Adverse events and clinical courses of the patients who underwent TCR-T cell transfer
| Patient . | Therapy-related adverse event . | Hematological effect . | WT1-mRNA level in PB on day 58 (day 0) (copy/mgRNA) . | Therapies after TCR-T cell transfer . |
|---|---|---|---|---|
| TCR-WT-02-M01 | Skin reaction (G1)* | Decrease of abnormal erythroblasts in PB | 5800 (2700) | Allogeneic HSCT |
| TCR-WT-03-M02 | None | Progressive disease | 29 000 (8400) | None |
| TCR-WT-06-E03 | Skin reaction (G1)* | Decrease of blasts in BM | 4300 (2000) | Cytarabine/aclarubicin |
| TCR-WT-07-M03 | Skin reaction*(G1), facial edema (G1), dermatitis (G1) | Progressive disease | 3600 (930) | Azacitidine |
| TCR-WT-08-N04 | Fever (G1), skin reaction (G1)* | Progressive disease | 76 000 (70 000) | Cytarabine/aclarubicin/G-CSF, lenalidomide, hydroxyurea |
| TCR-WT-09-N05 | Phlebitis (G2), arrhythmia (G1), skin reaction (G1)*, stomatitis (G1) | Progressive disease | 45 000 (17 000) | Hydroxyurea, cytarabine |
| TCR-WT-10-F02 | Skin reaction (G1)* | Stable disease | 17 000 (17 000) | None |
| TCR-WT-11-E04 | Skin reaction (G1)* | Decrease of blasts in BM | 6300 (6600) | None |
| Patient . | Therapy-related adverse event . | Hematological effect . | WT1-mRNA level in PB on day 58 (day 0) (copy/mgRNA) . | Therapies after TCR-T cell transfer . |
|---|---|---|---|---|
| TCR-WT-02-M01 | Skin reaction (G1)* | Decrease of abnormal erythroblasts in PB | 5800 (2700) | Allogeneic HSCT |
| TCR-WT-03-M02 | None | Progressive disease | 29 000 (8400) | None |
| TCR-WT-06-E03 | Skin reaction (G1)* | Decrease of blasts in BM | 4300 (2000) | Cytarabine/aclarubicin |
| TCR-WT-07-M03 | Skin reaction*(G1), facial edema (G1), dermatitis (G1) | Progressive disease | 3600 (930) | Azacitidine |
| TCR-WT-08-N04 | Fever (G1), skin reaction (G1)* | Progressive disease | 76 000 (70 000) | Cytarabine/aclarubicin/G-CSF, lenalidomide, hydroxyurea |
| TCR-WT-09-N05 | Phlebitis (G2), arrhythmia (G1), skin reaction (G1)*, stomatitis (G1) | Progressive disease | 45 000 (17 000) | Hydroxyurea, cytarabine |
| TCR-WT-10-F02 | Skin reaction (G1)* | Stable disease | 17 000 (17 000) | None |
| TCR-WT-11-E04 | Skin reaction (G1)* | Decrease of blasts in BM | 6300 (6600) | None |
PB, peripheral blood.
Reactions at peptide injection sites.
Cell kinetics in peripheral blood and bone marrow trafficking of WT1-specific TCR-T cells after transfer
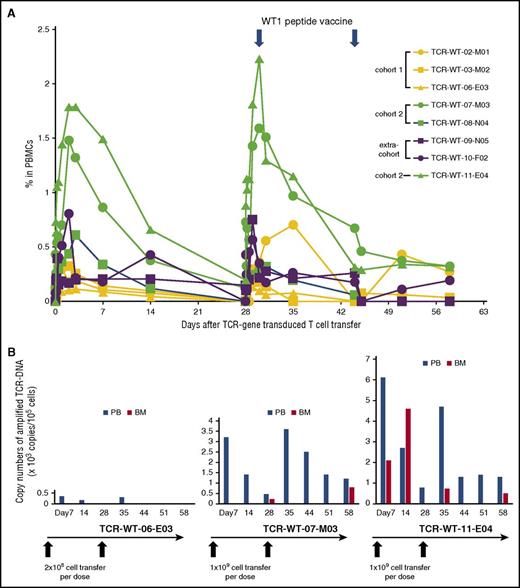
By analyzing TCR-transgene copies, infused cells were detected in peripheral blood in all 8 patients, appearing soon after transfer (Figure 1A). The peripheral levels of the TCR-T cells were dependent on the initial doses during the first 14 days, reaching peak and plateau levels on days 3 to 5, and then decreasing over the course of 14 days (Figure 1A). The transferred cells reached more than 0.5% to 2.0% of the PBMCs in patients of cohort 2 during the first 14 days, whereas in the case of cohort 1, these values reached 0.2% to 0.5% (supplemental Table 1). After the second cycle of the TCR-T cell transfer, which was followed by 2 vaccinations of WT1 peptide, 2 patients, TCR-WT-03-M01 and TCR-WT-07-M03, showed higher levels of TCR-T cells compared with cell numbers observed in the first cycle. In the other 6 patients, there seemed to be no differences between the 2 cycles. At the end of the study period on day 58, 5 patients showed persistent T cells (Figure 1A; supplemental Tables 2 and 3). TCR-WT-11-E04, who received 1 × 109 cell-dose per infusion, experienced the highest peripheral blood level of the TCR-T cells and showed hematological effects with blast numbers decreased in bone marrow (Figure 2A). In this patient, the peak level in the first phase was 1.79% of PBMCs, and in the second phase, the peak level was 2.23%, and 0.32% PBMCs on day 58 (supplemental Table 3). Because the proportion of CD8+ T-cells in the prepared gene-transduced cell product was 81.4% and the tetramer-positive cells constituted 29.5% (supplemental Table 1), 0.42%, 0.53%, and 0.008% of TCR-gene transduced CD8+ T cells circulated in peripheral blood at the first phase peak, the second phase peak, and on day 58, the end period of the study, respectively.
Cell kinetics in peripheral blood and bone marrow trafficking of WT1-specific TCR-T cells after the cell transfer into 8 patients. (A) Panels show kinetics of 3 patients who received 2 × 108 cells per infusion (cohort 1), 3 patients who received 1 × 109 cells (cohort 2), and 2 patients, TCR-WT-09-N05 and TCR-WT-10-F02, who received 9.7 × 108 cells (first cycle), 5.6 × 108 cells (second cycle), and 6.5 × 108 cells per infusion (extracohort), respectively. PB was collected at baseline and at predetermined times during a period of 58 days. DNA samples were extracted from the PBMCs, and TCR gene copy numbers were measured by qPCR. (B) Bone marrow trafficking of WT1-TCR-T cells in 3 patients compared with those in the peripheral blood at the same times is presented. The detection limit of the transduced cells is 100 copies/105 cells.
Cell kinetics in peripheral blood and bone marrow trafficking of WT1-specific TCR-T cells after the cell transfer into 8 patients. (A) Panels show kinetics of 3 patients who received 2 × 108 cells per infusion (cohort 1), 3 patients who received 1 × 109 cells (cohort 2), and 2 patients, TCR-WT-09-N05 and TCR-WT-10-F02, who received 9.7 × 108 cells (first cycle), 5.6 × 108 cells (second cycle), and 6.5 × 108 cells per infusion (extracohort), respectively. PB was collected at baseline and at predetermined times during a period of 58 days. DNA samples were extracted from the PBMCs, and TCR gene copy numbers were measured by qPCR. (B) Bone marrow trafficking of WT1-TCR-T cells in 3 patients compared with those in the peripheral blood at the same times is presented. The detection limit of the transduced cells is 100 copies/105 cells.
Clinical courses of TCR-WT-11-E04 and TCR-WT-06-E03 after the WT-specific TCR-T cell transfer. (A) The patient received 2 cycles of the TCR–T-cell therapy, and the second infusion was followed by 2 doses of WT1 peptide vaccination. One month after the second cycle, peripheral blood cell counts increased in association with a decrease in bone marrow blast counts. The patient did not require further red blood cell transfusion. (B) The patient received 2 cycles of the TCR-T cell therapy, and the second infusion was followed by 2 doses of WT1 peptide vaccination. After the second cycle, peripheral blood cell counts increased in association with decreased blast counts in bone marrow. Hb, hemoglobin; neut., neutrophil; Plt, platelet; RBC, red blood cell; WBC, white blood cell.
Clinical courses of TCR-WT-11-E04 and TCR-WT-06-E03 after the WT-specific TCR-T cell transfer. (A) The patient received 2 cycles of the TCR–T-cell therapy, and the second infusion was followed by 2 doses of WT1 peptide vaccination. One month after the second cycle, peripheral blood cell counts increased in association with a decrease in bone marrow blast counts. The patient did not require further red blood cell transfusion. (B) The patient received 2 cycles of the TCR-T cell therapy, and the second infusion was followed by 2 doses of WT1 peptide vaccination. After the second cycle, peripheral blood cell counts increased in association with decreased blast counts in bone marrow. Hb, hemoglobin; neut., neutrophil; Plt, platelet; RBC, red blood cell; WBC, white blood cell.
Bone marrow samples were obtained from 3 patients after TCR-T cell transfer (TCR-WT-06-E03, TCR-WT-07-M03, and TCR-WT-11-E04). In 2 patients, the TCR-T cells were detected. In TCR-WT-11-E04, WT1-specific TCR-T cells constituted a higher proportion than PBMCs on day 14 (Figure 1B), which indicated that the TCR-T cells preferentially migrated to the bone marrow.
Immune reactivity against WT1 peptide
We collected peripheral blood T cells from the patients, stimulated these cells in vitro with WT1 peptide, and performed tetramer and ELISPOT assays.
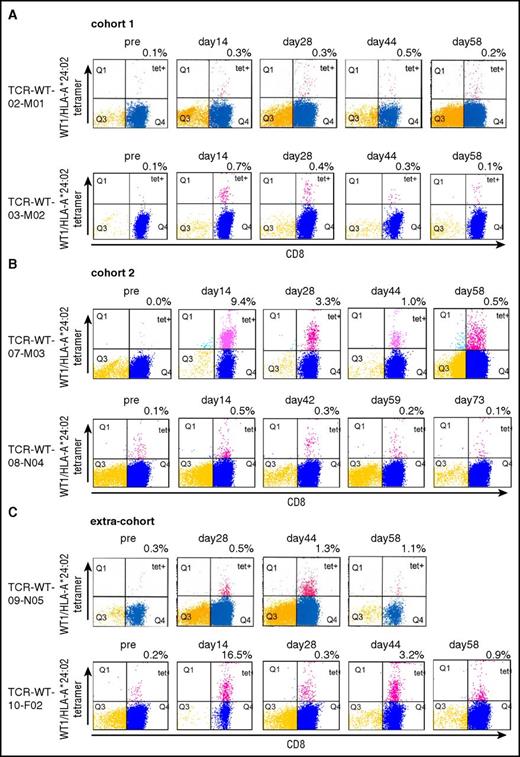
In cohort 1, WT1/HLA:A*24:01 tetramer-positive CD8+ T cells were detected in 2 patients between days 14 and 58, at levels of 0.5% or 0.7% in the peptide-stimulated T cells (Figure 3A). In the 2 patients in cohort 2 or extracohort, higher rates of the tetramer-positive CD8+ T cells compared with those in cohort 1 were detected with 9.4% and 16.5% in the peptide-stimulated T cells in TCR-WT-07-M03 and TCR-WT-10-F02, respectively. In the 2 patients who received higher doses of the tetramer-positive CD8+ T cells, TCR-WT-07-M03 and TCR-WT-10-F-02, higher frequency of the tetramer-positive cells were detected in peripheral blood compared with those who received lower doses of the tetramer-positive CD8+ T cells, TCR-WT-08-N04 and TCR-WT-09-N05. (supplemental Table 1; Figure 3B-C). In 2 of the 4 patients, TCR-WT-07-M03 and TCR-WT-10-F02, the peptide-reactive CD8+ T cells were still detected on day 58 (Figure 3B-C).
Tetramer analysis of the TCR-T cells at pre- and posttransfer times in peripheral blood. (A) PBMCs collected from 2 patients in cohort 1 (2 × 108 cells per infusion), before and after transfer of the TCR-T cells. CD8+ T cells were selected, stimulated in vitro with WT1 peptide, and assayed for tetramers of WT1 peptide/HLA-A*24:02. (B) PBMCs collected from 2 patients in cohort 2 (1 × 109 cells per infusion), before and after transfer of the TCR-T cells. (C) PBMCs collected from 2 patients who were allocated to cohort 2 (1 × 109 cells per infusion) and whose cells failed to proliferate to the target T-cell number, before and after transfer of the TCR-T cells.
Tetramer analysis of the TCR-T cells at pre- and posttransfer times in peripheral blood. (A) PBMCs collected from 2 patients in cohort 1 (2 × 108 cells per infusion), before and after transfer of the TCR-T cells. CD8+ T cells were selected, stimulated in vitro with WT1 peptide, and assayed for tetramers of WT1 peptide/HLA-A*24:02. (B) PBMCs collected from 2 patients in cohort 2 (1 × 109 cells per infusion), before and after transfer of the TCR-T cells. (C) PBMCs collected from 2 patients who were allocated to cohort 2 (1 × 109 cells per infusion) and whose cells failed to proliferate to the target T-cell number, before and after transfer of the TCR-T cells.
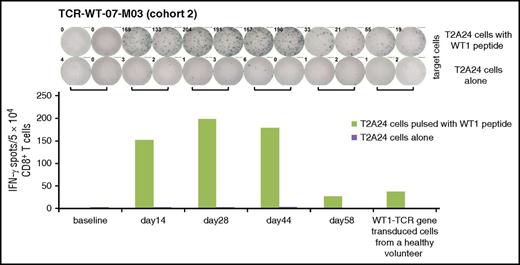
In the peptide-stimulated CD8+ T cells, definite IFN-γ releasing T cells were detected in 1 patient, TCR-WT-07-M03, by ELISPOT. About 0.3% to 0.4% CD8+ T cells responded to WT1-peptide between days 14 and 44, and decreased by day 58 (Figure 4).
Reactivity to WT1 peptide in PBMCs after the TCR-T cells transfer. PBMCs collected from TCR-WT-07-M03 in cohort 2, before and after transfer of the TCR-T cells. CD8+ T-cells were selected, stimulated in vitro with WT1 peptide, and subjected to an ELISPOT assay. The target T cells were T2A24 cells pulsed with WT1 peptide. T2A24 cells alone were used as control targets, and WT1-specific TCR-T cells from a healthy volunteer were used as positive effector cells.
Reactivity to WT1 peptide in PBMCs after the TCR-T cells transfer. PBMCs collected from TCR-WT-07-M03 in cohort 2, before and after transfer of the TCR-T cells. CD8+ T-cells were selected, stimulated in vitro with WT1 peptide, and subjected to an ELISPOT assay. The target T cells were T2A24 cells pulsed with WT1 peptide. T2A24 cells alone were used as control targets, and WT1-specific TCR-T cells from a healthy volunteer were used as positive effector cells.
Clinical course after transfer of WT1-specific TCR-T cells
During the 58-day study period, 4 patients developed progressive disease (Table 2). Two patients, TCR-WT-06-E03 and TCR-WT-11-E04, showed transient decreases in blast counts in bone marrow associated with peripheral blood cell recovery (Figure 2A-B). In TCR-WT-11-E04 during the study period, the blasts transiently increased from 10.7% at baseline to 24.8%, and then declined to 7.9% at the end of the study period (Figure 2A). In patient TCR-WT-06-E03, we observed blast decline from 24.8% at preinfusion of the TCR-T cells to 18.4% postinfusion (Figure 2B). We also observed transient decreases in erythroblasts soon after the TCR-T cell transfer in TCR-WT-02-M01 (Table 2) (data not shown). We measured peripheral WT1 mRNA levels at study entry, day 0, day 28, and day 58. We did not see an overall decline of peripheral WT1 mRNA in any of the cases (supplemental Figure 2).
Five patients survived longer than 12 months, and 1 patient is still alive to date. This patient, TCR-WT-02-M01, underwent allogenic cord blood stem cell transplant in 2 months after the TCR-T cell transfer. Four of 5 patients who had persistent T cells at the end of the study period survived more than 12 months, whereas in 3 patients from whom no more TCR-T cells were detected on day 58, only 1 patient, TCR-WT-06-E03, who exhibited transient blast decline, survived beyond 12 months. The survival durations ranged from 2.7 to 39.4 months, with a median of 15.9 months (supplemental Figure 3).
Discussion
To our knowledge, this study is the first clinical trial involving antigen-receptor gene-transduced T-cell transfer targeting the WT1 antigen. Our trial demonstrated that WT1-specific TCR-T cells that were produced by ex vivo culture of polyclonal peripheral T cells survived in vivo and retained the capacity to mount immune responses to WT1, and that they further provided transient antileukemic effects in patients with refractory AML.
WT1 antigen is known to be expressed on a wide variety of malignant tissues, and in particular, blast cells in AML and MDS express it at high levels. As an immunological therapeutic target, several studies have been reported, and WT1 epitope peptides recognized by CTL have been identified.5-7 The peptides of WT1126-134 with HLA-A*02:01 and WT1235-243 with HLA-A*24:02 restriction have been investigated as potential cancer vaccines, in which no serious toxicities were observed, and some cases showed clinical responses, but most patients failed to benefit.17-20 Another clinical study on WT1 targeting cell therapy was previously reported.21 In this clinical trial, stem cell transplant donor-derived T cell clones specific to WT1 antigen were transferred to patients with leukemia who had relapsed after allogeneic transplant. In 2 of 11 patients, antileukemic activity was shown without adverse events. These findings suggested that even though WT1 peptides could be sufficiently immunogenic so as to generate effector T cells in vivo, much greater numbers of T cells are required to induce visible clinical effects in patients with leukemia.
WT1 antigen is also expressed in certain normal tissues, including renal glomerular podocytes, mesothelial cells, and hematopoietic stem cells.22 In targeting WT1 by using specific reactive T cells, caution should be taken regarding potential toxicity to normal tissues that also express WT1. Thus, we preclinically tested the toxicity of WT1-specific TCR-T cells on normal hematopoietic stem cells and renal podocytes. We found that the TCR-T cells did not disrupt human hematopoiesis in vitro or in a NOD-SCID mouse model in vivo.8 Moreover, we observed that they exerted cytotoxic effects against podocytes in vitro; however, they did not damage podocytes in vivo.23 On the basis of these findings, we planned to investigate the safety of the TCR-T cells in this clinical trial, and we did not detect any toxicity to normal tissues. At this time, the major approach to treat acute lymphoblastic leukemia and other B-cell malignancies by antigen-receptor-engineered T cells involves CAR-T cell therapy; in particular, CD19 is the most promising target antigen.24,25 CD19-CAR technology can confer to T cells high-binding capacity of the antibody Fab portion, which enables T-cell therapy to efficiently destroy CD19+ tumor cells, as well as that by normal B cells. From recent reports of TCR-gene engineered T cells, mutated or mouse-derived high-affinity TCR often induces severe on-target toxicities.26,27 In the current trial, we used WT1-specific TCR that was derived from a healthy individual and was nonmutated wild-type.5,8 If high-affinity TCR or CAR technology that recognizes major histocompatibility complex (MHC)–peptide complexes on the cell surface were to be applied to WT1, much caution should be applied regarding the risk for normal tissue toxicity induced by the T cells, because high-affinity receptors may exert remarkable effects on normal tissues that express small amounts of tumor antigen.27,28 In addition, it is theoretically possible that a TCR could induce toxicity by reacting to peptides from a different antigen and includes sequences similar to the target peptide. Indeed, affinity-enhanced MAGE-A3-specific TCR-T cells caused serious cardiac toxicity with unexpected recognition of titin, a cardiac muscle antigen, in patients treated for melanoma and myeloma.29,30 Despite the notions of definite WT1 expression on renal podocytes, mesothelial cells, and hematopoietic stem cells, there has been no detailed information concerning WT1 expression on other normal tissues. It would be important to strike a balance between the risk for normal organ toxicity and the high immunogenic effects induced by high-affinity antigen receptors, and more important, extensive exploration of on-target normal tissue toxicities and potential reactivity to analogous peptides from different antigens should be planned before a first-in-human clinical trial.
This clinical trial was designed as a cell-dose escalation study, commencing at 2 × 108 cells per infusion to 1 × 109 cells, and in autologous lymphocyte preparations, 2 of 5 patients’ cells failed to reach target levels of proliferation. The proliferation patterns were variable among the individuals, probably because of influences of prior chemotherapies. In patients with MDS, some lymphocytes are involved in MDS clonal differentiation; abnormal blasts also often infiltrate the bone marrow, and consequently, normal hematopoiesis may be dysregulated. Therefore, we should consider suitable candidates in whom autologous TCR-T cells can be proliferated ex vivo to the desired cell dose levels. Otherwise, healthy donor-derived TCR-T cells could be applicable if we overcome the risk for allogeneic immune reactions such as graft-versus-host disease. In clinical trials with T cells genetically engineered to express tumor-reactive receptors, persistence of the infused cells seems to be necessary.2,25 We measured retroviral DNA copies in PBMCs soon after the TCR-T cell transfer and throughout the final observation period after the second transfer, which enabled us to see detailed in vivo cell kinetics of the transferred T cells. We found cell dose-dependent kinetics in peripheral blood, forming plateaus between days 2 and 5 and gradually declining afterward. The second cycle was followed by 2 vaccinations of WT1 peptide, in which we did not see significant differences with the first cycle. Although we initially expected higher cell levels in the second cycle, we did not demonstrate the significance of the peptide vaccine as part of a combination therapy as long as we observed the levels in peripheral blood. In our previous study of MAGE-A4 targeting TCR-T cell transfer, we observed that short MAGE-A4 peptides induced decreases of the TCR-T cell level in peripheral blood.15 Together with the present observations, we are able to explain that cognate peptide vaccines might have induced T cell apoptosis, consequently reducing the total number of TCR-transduced T cells.31,32
At the end of the study period, 5 of 8 patients still had detectable levels of the TCR-T cells, with levels ranging from 0.03% to 0.32% of the total PBMC population. We did not measure long-term peripheral levels of the T cells thereafter, but we can suppose that some of the T cells might survive and persistently react with leukemic cells.
Two patients showed transient decreases of blasts in bone marrow associated with recovery of hematopoiesis. One of the patients received the higher dose of 1 × 109 T cells, and the TCR-T cells in PBMCs appeared at the highest levels among all the 8 patients, which still persisted at 0.32% on day 58. In addition, the TCR-T cells were detected in the bone marrow at a higher level than that in peripheral blood. We can now understand that the T cells preferentially migrated to the bone marrow and constantly reacted with leukemic cells, eventually manifesting clinical effects.
The present clinical study demonstrated that WT1-specific TCR-T cells prepared ex vivo using cultured autologous lymphocytes from peripheral blood survived in patients with AML and MDS, and displayed immune reactivity to WT1. The hematologic efficiency was not yet definite in our patients, partly because of the interindividual nature of the TCR–T-cell preparation, and possibly also because of the binding affinity using a wild-type WT1-specific TCR. Because a TCR affinity to a peptide–MHC complex is essential for achieving cytotoxic T-cell function, especially to tumor antigens rather than pathogen-derived peptides, affinity-enhancement of the WT1-specific TCR using a sequence-optimization would be necessary.33 In addition, this immunotherapy targeting WT1 antigen for AML and MDS would be more clinically beneficial, if we target a more suitable subpopulation. For example, subtypes of AML and MDS that are sensitive to WT1-specific TCR-T cells should be explored. Another strategy would be targeting an antigen other than WT1. An antibody recognizing a complex of PRAME-derived peptide/HLA class I was investigated.34 The other one would be PR1, which was clinically investigated as a peptide vaccine in patients with AML.35 Receptor-redirected CAR-T or TCR-T cells targeting these antigens could be promising. Furthermore, combining a preconditioning regimen to deplete lymphocytes in a host and/or an immune-checkpoint inhibitor, such as anti-programmed cell death 1 antibody, would be discussed in planning a future clinical trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all coworkers from all units of Mie University Hospital, Ehime University Hospital, Fujita Health University Hospital, and Nagoya University Hospital for their skillful assistance in making this trial run successfully and for the support they provided to the patients under their care. The authors thank all coworkers from FiveRings, Co. Ltd., and TakaraBio Inc. for operating and analyzing this study. The authors also thank Kaoru Hirai, Hiroshi Miyamoto (FiveRings, Co. Ltd.), Shuichi Takahashi, Ichiro Kawashima, and Kazutoh Takesako (TakaraBio Inc.) for their special contributions to operating this clinical trial. Sahoko Sugino and Junko Nakamura (Mie University) provided technical assistance in the tetramer and ELISPOT assay. This study was technologically supported by TakaraBio Inc.
This research is supported by the Translational Research Network Program (grant no.16lm0103009j0005) from Japan Agency for Medical Research and Development. It was partly funded by grants-in-aid for scientific research from the Japan Society for the Promotion of Science.
Authorship
Contribution: I.T., S.K., Y.M., H.F., T.N., Y.A., H.I., K.T., S.T., M. Murata, Y.I., M. Masuya, and H.S. designed, analyzed, and interpreted the clinical studies and wrote the manuscript; N.I., T.K., S.O., D.T., H.C., I.N., and J.M. provided technical assistance of cell preparation and cell kinetics; and T.N., N.E., M.Y., N.K., and H.S. supervised the research.
Conflict-of-interest disclosure: N.I., T.K., S.O., D.T., H.C., I.N., and J.M. are employees of TakaraBio Inc. TakaraBio Inc. provided the Department of Immuno-Gene Therapy in Mie University with funding resources. S.K., Y.M., and H.S. are members of this department. The remaining authors declare no competing financial interests.
Correspondence: Shinichi Kageyama, Department of Immuno-Gene Therapy, Mie University Graduate School of Medicine, 2-174, Edobashi, Tsu, Mie 514-8507, Japan; e-mail kageyama@clin.medic.mie-u.ac.jp; and Hiroshi Shiku, Department of Immuno-Gene Therapy, Mie University Graduate School of Medicine, 2-174, Edobashi, Tsu, Mie 514-8507, Japan; e-mail shiku@clin.medic.mie-u.ac.jp.