Key Points
An inactivating point mutation in the hemITAM motif of murine CLEC-2 reproduces the lymphatic defects seen in CLEC-2–deficient mice.
CLEC-2 contributes to thrombus stability in vivo independently of hemITAM signaling.
Abstract
C-type lectin-like receptor 2 (CLEC-2) is a platelet receptor that is critical during development in blood-lymph separation and implicated in thrombus stability in thrombosis and hemostasis. It is the only known platelet activatory receptor that participates in both of these aspects of platelet function, and it is the only one to signal through a hemi-immunoreceptor tyrosine–based activation motif (hemITAM). Current investigations into the function of CLEC-2 in vivo have focused on knockout (KO) studies in which both the receptor and its signaling are deleted, making it impossible to explore the possible signaling-independent functions of the receptor, which are indicated by its only known physiological ligand, podoplanin, being an integral membrane protein. In this report, we present a novel knockin mouse model that maintains the expression of a CLEC-2 receptor that cannot signal through its hemITAM (Y7A KI). Remarkably, this mouse phenocopies the blood-lymphatic mixing and lethality of CLEC-2 KO models, but not their hemostatic/thrombotic defect. However, treatment of Y7A KI mice with Fab′ fragments of the function-blocking anti–CLEC-2 antibody, INU1, resulted in a thrombus formation defect in vivo and ex vivo, revealing a hemITAM signaling–independent role for CLEC-2 in hemostasis and thrombosis.
Introduction
Platelets play an essential role in both development and hemostasis, and C-type lectin-like receptor 2 (CLEC-2) is the major platelet activatory surface receptor known to contribute to both of these platelet functions.1-3 Furthermore, CLEC-2 is the only known platelet receptor to signal through a hemi-immunoreceptor tyrosine–based activation motif (hemITAM), and its only known physiological ligand, the transmembrane protein podoplanin, is not expressed in the vasculature in healthy conditions and is not known to participate in primary hemostasis.4,5 The function of CLEC-2 in vivo has been primarily investigated through the generation of knockout (KO) mouse models and mice in which the receptor has been depleted from circulating platelets by injection of the anti–CLEC-2 antibody, INU1. Both of these approaches lead to a complete loss of the receptor and therefore its signaling in platelets.1-3,6-11 These models have revealed the importance of platelet-expressed CLEC-2 in the separation of the blood and lymphatic systems and indicated a potential role for the receptor in thrombus stability in hemostasis and thrombosis. However, due to their nature, these studies have been unable to investigate any hemITAM-independent functions of CLEC-2 or dissociate its role in development from its function in hemostasis.
Study design
Animals
Platelet preparation, flow cytometry, and western blotting
Platelet isolation, subsequent flow cytometry, and western blotting experiments were performed as described previously.1,6 Antibodies used in western blotting were as follows: anti–CLEC-2 (clone INU2, generated in house)6 ; anti-Syk and anti-Lyn (clone C13F9) (Cell Signaling Technology); and anti-Fyn (clone FYN3) and anti-PLCγ2 from Santa Cruz Biotechnology.
Ex vivo flow chamber assay
In vivo thrombosis assays
Chemical injury of the mesenteric arterioles and mechanical injury of the aorta were as previously described.14 Where used, INU1 Fab′ fragments at 2 µg/g mouse were injected retro-orbitally 5 minutes before vessel injury.
Statistical analysis
The data presented are means ± standard deviations and, unless otherwise stated, were analyzed by unpaired Student t test. P values <.05 were considered statistically significant.
Results and discussion
Constitutive and chimeric CLEC-2 Y7A knockin mice have a defect in blood-lymph separation
Constitutive Y7A CLEC-2 knockin (Y7A KI) mice were generated by insertion of the point mutation into the first exon of the Clec1b gene (supplemental Figure 1, available on the Blood Web site). Homozygous Y7A KI embryos showed signs of hemorrhage in the brain at E12.5 and by E14.5 edema and blood-lymphatic mixing in the developing skin lymphatics (Figure 1A), closely resembling the developmental phenotype of CLEC-2 KO mouse models.2,3,8,10,11 Because the constitutive Y7A KI mice died shortly after birth (supplemental Tables 1 and 2; supplemental Figure 2), we generated fetal liver chimeric mice. Again resembling (chimeric) CLEC-2 KO mice,2,3,8,11 chimeric Y7A KI mice developed progressive blood-lymphatic mixing, with blood filling the mesenteric lymphatics (Figure 1B; supplemental Figure 3). These data confirmed the critical role of CLEC-2 hemITAM signaling to prevent blood filling of the lymphatics during development and postirradiation in adult mice.
The Y7A point mutation in the hemITAM motif of CLEC-2 abolishes platelet responses to CLEC-2 agonists and reproduces the lymphatic defects seen in CLEC-2–deficient mice. (A) Embryos from time-mated Y7A knockin (KI) heterozygous crosses at E12.5 and E14.5 show hemorrhages in the brain and edema and lymphatic blood-filling in the Y7A KI embryos, respectively. Representative pictures of wild-type and Y7A KI embryos from 5 different litters at each embryo stage. Original magnifications ×3.0 (upper panels [E12.5]) and ×1.8 (lower panels [E14.5]). (B) Adult mice transplanted with Y7A KI fetal liver cells after irradiation developed blood-filling of the lymphatics. Representative images of the mesenteric blood and lymphatic vessels are shown. Original magnification ×25. (C) CLEC-2 surface expression was maintained on Y7A KI platelets at levels comparable with wild-type platelets, as measured by flow cytometry. There was a small but significant reduction in the CLEC-2 surface levels in Y7A KI platelets (*P < .05), however, this was due to the anti–CLEC-2 antibody used (fluorescein isothiocyanate–conjugated INU1 immunoglobulin G) activating wild-type1 but not Y7A KI platelets during the staining process, thus releasing a small intracellular pool of CLEC-2 to the platelet surface in wild-type platelets only. The results are presented as means with standard deviations, n = 20. (D) Western blotting of platelet lysates from wild-type and Y7A KI chimeric mice showed no difference in CLEC-2 protein levels or in the levels of the major hemITAM signaling proteins Syk, Lyn, Fyn, and PLCγ2. Blot represents 2 independent experiments. (E) Platelet integrin activation, measured as JON/A phycoerythrin antibody binding by flow cytometry, showed no differences in responses to the indicated agonists between wild-type and Y7A KI platelets from chimeric mice. (F) Y7A KI platelets showed no activation in response to rhodocytin, which could not be overcome by the exogenous addition of the secondary mediator agonist adenosine 5′-diphosphate (ADP) and the thromboxane mimetic U46619. Flow cytometry activation data are presented as means with error bars of standard deviations and are representative of 3 independent experiments, n = 4. (G) Washed platelet aggregometry experiments showed the complete loss of aggregation responses specifically to the CLEC-2 agonist rhodocytin in Y7A KI platelets (blue). Traces are representative of responses from n = 4 animals. (H) Pan-phosphotyrosine and phospho-specific western blots showed no induction of the hemITAM signaling cascade in CLEC-2 Y7A KI platelets after stimulation with 1 µg/ml rhodocytin. Representative western blots of 3 independent experiments. (I) There were no significant differences between the number of megakaryoctyes present per field of view (FOV) in chimeric wild-type and Y7A KI whole femora cyrosections; n = 3-6, minimum 5 fields of view per mouse. (J) There were no significant differences between chimeric wild-type and Y7A KI platelet counts as measured by flow cytometry. Counts were measured at 5 weeks posttransplantation, n = 12. CRP, collagen-related peptide; GMFI, geometric mean fluorescent intensity; Rhodo, rhodocytin.
The Y7A point mutation in the hemITAM motif of CLEC-2 abolishes platelet responses to CLEC-2 agonists and reproduces the lymphatic defects seen in CLEC-2–deficient mice. (A) Embryos from time-mated Y7A knockin (KI) heterozygous crosses at E12.5 and E14.5 show hemorrhages in the brain and edema and lymphatic blood-filling in the Y7A KI embryos, respectively. Representative pictures of wild-type and Y7A KI embryos from 5 different litters at each embryo stage. Original magnifications ×3.0 (upper panels [E12.5]) and ×1.8 (lower panels [E14.5]). (B) Adult mice transplanted with Y7A KI fetal liver cells after irradiation developed blood-filling of the lymphatics. Representative images of the mesenteric blood and lymphatic vessels are shown. Original magnification ×25. (C) CLEC-2 surface expression was maintained on Y7A KI platelets at levels comparable with wild-type platelets, as measured by flow cytometry. There was a small but significant reduction in the CLEC-2 surface levels in Y7A KI platelets (*P < .05), however, this was due to the anti–CLEC-2 antibody used (fluorescein isothiocyanate–conjugated INU1 immunoglobulin G) activating wild-type1 but not Y7A KI platelets during the staining process, thus releasing a small intracellular pool of CLEC-2 to the platelet surface in wild-type platelets only. The results are presented as means with standard deviations, n = 20. (D) Western blotting of platelet lysates from wild-type and Y7A KI chimeric mice showed no difference in CLEC-2 protein levels or in the levels of the major hemITAM signaling proteins Syk, Lyn, Fyn, and PLCγ2. Blot represents 2 independent experiments. (E) Platelet integrin activation, measured as JON/A phycoerythrin antibody binding by flow cytometry, showed no differences in responses to the indicated agonists between wild-type and Y7A KI platelets from chimeric mice. (F) Y7A KI platelets showed no activation in response to rhodocytin, which could not be overcome by the exogenous addition of the secondary mediator agonist adenosine 5′-diphosphate (ADP) and the thromboxane mimetic U46619. Flow cytometry activation data are presented as means with error bars of standard deviations and are representative of 3 independent experiments, n = 4. (G) Washed platelet aggregometry experiments showed the complete loss of aggregation responses specifically to the CLEC-2 agonist rhodocytin in Y7A KI platelets (blue). Traces are representative of responses from n = 4 animals. (H) Pan-phosphotyrosine and phospho-specific western blots showed no induction of the hemITAM signaling cascade in CLEC-2 Y7A KI platelets after stimulation with 1 µg/ml rhodocytin. Representative western blots of 3 independent experiments. (I) There were no significant differences between the number of megakaryoctyes present per field of view (FOV) in chimeric wild-type and Y7A KI whole femora cyrosections; n = 3-6, minimum 5 fields of view per mouse. (J) There were no significant differences between chimeric wild-type and Y7A KI platelet counts as measured by flow cytometry. Counts were measured at 5 weeks posttransplantation, n = 12. CRP, collagen-related peptide; GMFI, geometric mean fluorescent intensity; Rhodo, rhodocytin.
The Y7A mutation of CLEC-2 abolishes platelet responses to CLEC-2 agonists without affecting expression
Importantly, CLEC-2 expression was maintained on the platelet surface at levels comparable with wild-type, as were all other prominent platelet surface receptors (Figure 1C; supplemental Table 3) and major kinases and effector proteins of the hemITAM phosphotyrosine signaling cascade (Figure 1D). CLEC-2 Y7A KI platelets displayed normal responses to classic agonists, but a complete block in response to the CLEC-2 agonists rhodocytin and the activating anti–CLEC-2 antibody, INU1 (Figure 1E-H; supplemental Figure 4). Furthermore, megakaryocyte numbers in the bone marrow (Figure 1H; supplemental Figure 5) and platelet count and volume (Figure 1I and data not shown) were normal in the initial stages posttransplantation in Y7A KI mice, which supports the hypothesis that CLEC-2 hemITAM signaling is not critical for normal platelet production. Interestingly, platelet counts in Y7A KI mice significantly decreased over time as the blood-lymphatic mixing defect progressed to cause bloody ascites in the mice (supplemental Figure 6), suggesting that the reduced platelet counts seen in CLEC-2 KO mouse models3,7,15 are most likely due to the blood-lymphatic mixing and vascular integrity defects.16
Blocking the CLEC-2 ectodomain, but not abolished CLEC-2 hemITAM signaling, destabilizes platelet aggregate formation under flow
Remarkably, Y7A KI platelets formed aggregates on collagen under flow with similar kinetics and to a similar extent as wild-type platelets (Figure 2A), which was in clear contrast to the thrombus stability defect previously seen with CLEC-2–deficient platelets.1,2,7 To assess the contribution of the signal-dead receptor to thrombus stabilization, the assay was repeated in the presence of Fab′ fragments of the monoclonal antibody, INU1, which blocks the interaction of CLEC-2 and rhodocytin.1 Remarkably, the blockade of the CLEC-2 ectodomain resulted in a 50% decrease in platelet surface coverage with few 3-dimensional aggregates formed by Y7A KI platelets, which is reminiscent of the defect seen in CLEC-2–deficient platelets in this assay.1,2 This reduction in aggregate formation was not due to an effect of the Fab′ fragment on Y7A KI platelet activation, because responses to GPVI and GPCR agonists were maintained in the presence of the antibody (supplemental Figure 7).
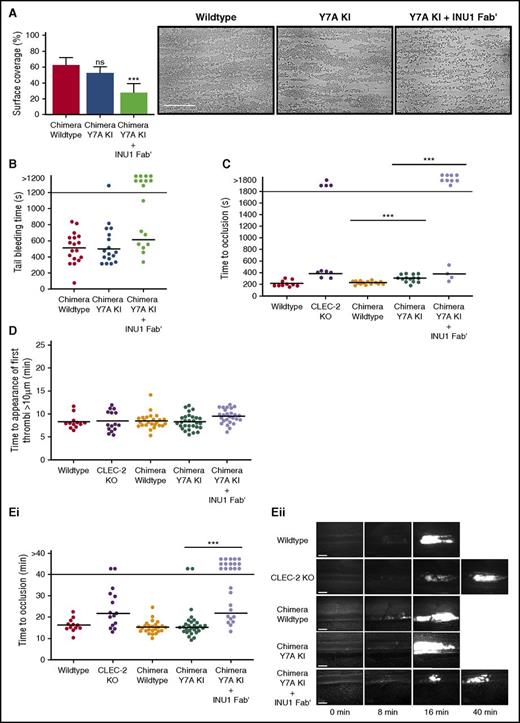
CLEC-2 contributes to thrombus stability in vitro and in vivo independently of hemITAM signaling. (A) The surface area of aggregates formed on collagen under flow conditions was significantly reduced in Y7A knockin (KI) chimeric samples only after preincubation with 5 µg/mL INU1 Fab′ fragments. Shear rate: 1000 s−1; data presented as means with error bars of standard deviations, n = 8. Representative images shown from the 5 fields of view analyzed per mouse. Scale bar represents 50 µm. (B) Y7A KI chimeric mice had normal tail bleeding times in comparison with wild-type mice, however, after injection of 2 µg/g of INU1 Fab′ fragments, Y7A KI mice had significantly destabilized hemostasis, with significantly more mice bleeding until the end of the observation period. The horizontal line denotes the mean time to cessation of bleeding, and each circle represents 1 animal. (C) Time to vessel occlusion in the mechanical injury of the aorta thrombosis model. Chimeric Y7A KI mice were able to form occlusive thrombi after injury, however, after injection of 2 µg/g of INU1 Fab′ fragments, Y7A KI mice had significantly destabilized thrombosis, reflecting the results of Pf4-cre CLEC-2 KO mice analyzed in parallel. The horizontal line denotes the mean time to vessel occlusion, and each circle represents 1 animal (D) There were no significant differences between any of the indicated mouse genotypes in the time to the first appearance of thrombi in the ferric chloride injury model of mesenteric arterioles. The horizontal line denotes the mean time to thrombi appearance, and each circle represents 1 animal. (Ei) Time to vessel occlusion in the ferric chloride injury model of mesenteric arterioles. (Eii) Representative images of injured vessels from the different mouse genotypes at the indicated time points after injury. There were no significant differences between vessel occlusion times in chimeric wild-type and Y7A KI mice, however, there was a significant reduction in vessels occluded within the observation period in chimeric Y7A KI mice treated with 2 µg/g of INU1 Fab′ fragments. This destabilization in thrombosis reflected the results of Pf4-cre CLEC-2 KO mice analyzed in parallel. The horizontal line denotes the mean time to vessel occlusion, each circle represents 1 arteriole, and 2 vessels were injured per animal. Platelets were visualized for microscopy with an anti-GPIX DyLight488-conjugated antibody derivative administered to the mice at the start of the experiment. Scale bars represent 20 µm. ***P < .001; ns, not significant.
CLEC-2 contributes to thrombus stability in vitro and in vivo independently of hemITAM signaling. (A) The surface area of aggregates formed on collagen under flow conditions was significantly reduced in Y7A knockin (KI) chimeric samples only after preincubation with 5 µg/mL INU1 Fab′ fragments. Shear rate: 1000 s−1; data presented as means with error bars of standard deviations, n = 8. Representative images shown from the 5 fields of view analyzed per mouse. Scale bar represents 50 µm. (B) Y7A KI chimeric mice had normal tail bleeding times in comparison with wild-type mice, however, after injection of 2 µg/g of INU1 Fab′ fragments, Y7A KI mice had significantly destabilized hemostasis, with significantly more mice bleeding until the end of the observation period. The horizontal line denotes the mean time to cessation of bleeding, and each circle represents 1 animal. (C) Time to vessel occlusion in the mechanical injury of the aorta thrombosis model. Chimeric Y7A KI mice were able to form occlusive thrombi after injury, however, after injection of 2 µg/g of INU1 Fab′ fragments, Y7A KI mice had significantly destabilized thrombosis, reflecting the results of Pf4-cre CLEC-2 KO mice analyzed in parallel. The horizontal line denotes the mean time to vessel occlusion, and each circle represents 1 animal (D) There were no significant differences between any of the indicated mouse genotypes in the time to the first appearance of thrombi in the ferric chloride injury model of mesenteric arterioles. The horizontal line denotes the mean time to thrombi appearance, and each circle represents 1 animal. (Ei) Time to vessel occlusion in the ferric chloride injury model of mesenteric arterioles. (Eii) Representative images of injured vessels from the different mouse genotypes at the indicated time points after injury. There were no significant differences between vessel occlusion times in chimeric wild-type and Y7A KI mice, however, there was a significant reduction in vessels occluded within the observation period in chimeric Y7A KI mice treated with 2 µg/g of INU1 Fab′ fragments. This destabilization in thrombosis reflected the results of Pf4-cre CLEC-2 KO mice analyzed in parallel. The horizontal line denotes the mean time to vessel occlusion, each circle represents 1 arteriole, and 2 vessels were injured per animal. Platelets were visualized for microscopy with an anti-GPIX DyLight488-conjugated antibody derivative administered to the mice at the start of the experiment. Scale bars represent 20 µm. ***P < .001; ns, not significant.
CLEC-2 ectodomain, but not hemITAM signaling, is required for hemostasis and thrombus stability
Importantly, chimeric Y7A KI mice displayed bleeding times comparable with chimeric wild-type mice in the filter paper tail bleeding assay, whereas treatment of the mice with INU1 Fab′ led to defective hemostasis (Figure 2B). A similar result was also seen in the 2 following in vivo thrombosis models: mechanical injury of the abdominal aorta (Figure 2C) and chemical injury of mesenteric arterioles by topical application of ferric chloride (Figure 2D-E). In both models, platelet-specific conditional CLEC-2 KO mice7 were analyzed in parallel with chimeric Y7A KI mice to allow direct comparison. Control experiments with chimeric Y7A KI and CLEC-2 KO mice injected with INU1 Fab′ confirmed that INU1 Fab′ does not exert any detectable nonspecific effects on platelet count, CLEC-2 surface expression, or thrombosis (supplemental Figures 8 and 9). The defect in thrombus formation in both CLEC-2 KO mice and INU1 Fab′–treated chimeric Y7A KI mice was not due to impaired thrombosis initiation (Figure 2D), but a destabilization of formed thrombi that resulted in frequent embolization, thus preventing vessel occlusion within the observation period (supplemental Videos 1-3).
In this study, we have generated a novel CLEC-2 KI mouse model that maintained the surface expression of a signaling-dead CLEC-2 receptor. The close developmental phenocopy of this mouse to other constitutive CLEC-2 knockout models is not unexpected given the clear contribution of CLEC-2 signaling to the prevention of lymphatic blood-filling, as indicated by the Syk, SLP-76, and PLCγ2 knockout mice.3,11,17-19 However, this new model has identified an as yet undescribed signaling-independent function of CLEC-2. This is the first evidence that CLEC-2 does not contribute to thrombus stability through a hemITAM signaling cascade. The antibody Fab′ fragment used to block mutant CLEC-2 in this study has been previously shown to block the ability of rhodocytin to activate platelets,1 indicating that the respective domain of CLEC-2 may be important for the observed thrombus-stabilizing role of the receptor. Further studies will be required to identify the blood-borne ligand/counterreceptor of CLEC-21,2,7 through which it mediates its signaling-independent adhesive role in thrombosis and hemostasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Sebastian Dütting and Julia Volz for contributing their technical expertise to some of the work in this manuscript.
This work was supported by Deutsche Forschungsgemeinschaft grants SFB688 (B.N.) and Eb177/13-1 (J.A.E.) and research fellowship CH 1734/1-1 (D.C.), and by British Heart Foundation grant RG/13/18/30563 (S.P.W.).
Authorship
Contribution: E.J.H. performed the experiments, analyzed the data, and wrote the manuscript; K.W., I.C.B., S.B., and D.S. performed the experiments and analyzed the data; J.A.E. contributed vital reagents and proofread the manuscript; D.C. and S.P.W. wrote the manuscript; and B.N. supervised the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.J.H. is Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom.
The current affiliation for D.C. is Immune Disease Institute, Program in Cellular and Molecular Medicine, Children’s Hospital Boston and Department of Pediatrics, Harvard Medical School, Boston, MA.
Correspondence: Bernhard Nieswandt, Institute of Experimental Biomedicine, University Hospital and Rudolf Virchow Center, University of Würzburg, Building D15, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.

![Figure 1. The Y7A point mutation in the hemITAM motif of CLEC-2 abolishes platelet responses to CLEC-2 agonists and reproduces the lymphatic defects seen in CLEC-2–deficient mice. (A) Embryos from time-mated Y7A knockin (KI) heterozygous crosses at E12.5 and E14.5 show hemorrhages in the brain and edema and lymphatic blood-filling in the Y7A KI embryos, respectively. Representative pictures of wild-type and Y7A KI embryos from 5 different litters at each embryo stage. Original magnifications ×3.0 (upper panels [E12.5]) and ×1.8 (lower panels [E14.5]). (B) Adult mice transplanted with Y7A KI fetal liver cells after irradiation developed blood-filling of the lymphatics. Representative images of the mesenteric blood and lymphatic vessels are shown. Original magnification ×25. (C) CLEC-2 surface expression was maintained on Y7A KI platelets at levels comparable with wild-type platelets, as measured by flow cytometry. There was a small but significant reduction in the CLEC-2 surface levels in Y7A KI platelets (*P < .05), however, this was due to the anti–CLEC-2 antibody used (fluorescein isothiocyanate–conjugated INU1 immunoglobulin G) activating wild-type1 but not Y7A KI platelets during the staining process, thus releasing a small intracellular pool of CLEC-2 to the platelet surface in wild-type platelets only. The results are presented as means with standard deviations, n = 20. (D) Western blotting of platelet lysates from wild-type and Y7A KI chimeric mice showed no difference in CLEC-2 protein levels or in the levels of the major hemITAM signaling proteins Syk, Lyn, Fyn, and PLCγ2. Blot represents 2 independent experiments. (E) Platelet integrin activation, measured as JON/A phycoerythrin antibody binding by flow cytometry, showed no differences in responses to the indicated agonists between wild-type and Y7A KI platelets from chimeric mice. (F) Y7A KI platelets showed no activation in response to rhodocytin, which could not be overcome by the exogenous addition of the secondary mediator agonist adenosine 5′-diphosphate (ADP) and the thromboxane mimetic U46619. Flow cytometry activation data are presented as means with error bars of standard deviations and are representative of 3 independent experiments, n = 4. (G) Washed platelet aggregometry experiments showed the complete loss of aggregation responses specifically to the CLEC-2 agonist rhodocytin in Y7A KI platelets (blue). Traces are representative of responses from n = 4 animals. (H) Pan-phosphotyrosine and phospho-specific western blots showed no induction of the hemITAM signaling cascade in CLEC-2 Y7A KI platelets after stimulation with 1 µg/ml rhodocytin. Representative western blots of 3 independent experiments. (I) There were no significant differences between the number of megakaryoctyes present per field of view (FOV) in chimeric wild-type and Y7A KI whole femora cyrosections; n = 3-6, minimum 5 fields of view per mouse. (J) There were no significant differences between chimeric wild-type and Y7A KI platelet counts as measured by flow cytometry. Counts were measured at 5 weeks posttransplantation, n = 12. CRP, collagen-related peptide; GMFI, geometric mean fluorescent intensity; Rhodo, rhodocytin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/20/10.1182_blood-2017-03-771907/4/m_blood771907f1.jpeg?Expires=1767736885&Signature=Sv2AdduW6w8EM4C~C-XAYgAyRv7Dn7swJx~tZwATH8f4nRMTpQGDOvykZhwCjd16-x2QoRiV5G6qAw3MSafnj9va1gbsQLbgHPIlzxFNZCzN~0Le4j7L2EnKs6uvCvT54LVuPATKHdcbf7LT~I5SKPybYRNUKbc2JFSLADZxiwEF3o5pONlUDrxEMIRDPjnoWGCS5kc-5uvVSj1P5r07pT-tihgek3F79H3H-gUQMnJsSzIUYf9Qnt9VsgOSYEjd8WVcQ2CM6kU0s4wCcuXyu~9chokX77BF-6eLlE77ovLpd6eE~Jh5ZU6hiI1CLX2wFutNuSQuEiVT-acR7NkVhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal