Key Points
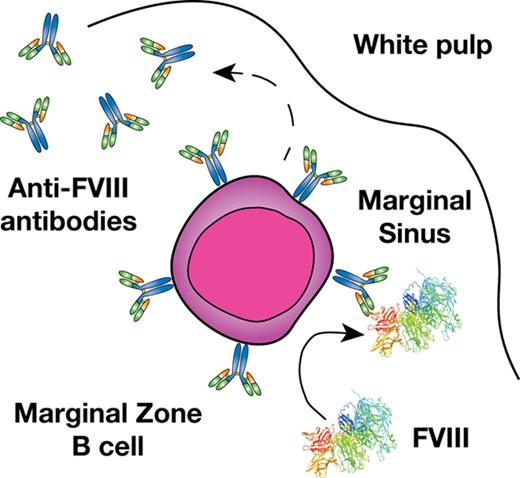
FVIII colocalizes with MZ B cells following infusion into hemophilia A mice.
Depletion of MZ B cells prevents FVIII inhibitor development in hemophilia A mice.
Abstract
Although factor VIII (FVIII) replacement therapy can be lifesaving for patients with hemophilia A, neutralizing alloantibodies to FVIII, known as inhibitors, develop in a significant number of patients and actively block FVIII activity, making bleeding difficult to control and prevent. Although a variety of downstream immune factors likely regulate inhibitor formation, the identification and subsequent targeting of key initiators in inhibitor development may provide an attractive approach to prevent inhibitor formation before amplification of the FVIII immune response occurs. As the initial steps in FVIII inhibitor development remain incompletely understood, we sought to define early regulators of FVIII inhibitor formation. Our results demonstrate that FVIII localizes in the marginal sinus of the spleen of FVIII-deficient mice shortly after injection, with significant colocalization with marginal zone (MZ) B cells. FVIII not only colocalizes with MZ B cells, but specific removal of MZ B cells also completely prevented inhibitor development following FVIII infusion. Subsequent rechallenge with FVIII following MZ B-cell reconstitution resulted in a primary antibody response, demonstrating that MZ B-cell depletion did not result in FVIII tolerance. Although recipient exposure to the viral-like adjuvant polyinosinic:polycytidylic acid enhanced anti-FVIII antibody formation, MZ B-cell depletion continued to display similar effectiveness in preventing inhibitor formation following FVIII infusion in this inflammatory setting. These data strongly suggest that MZ B cells play a critical role in initiating FVIII inhibitor formation and suggest a potential strategy to prevent anti-FVIII alloantibody formation in patients with hemophilia A.
Introduction
Hemophilia A is an X-linked bleeding disorder characterized by a deficiency or absence of the blood coagulation protein, factor VIII (FVIII). Patients with hemophilia A depend on FVIII replacement by IV infusion for acute bleeding episodes or prevention of bleeding.1 The most significant complication of factor replacement therapy is the development of neutralizing immunoglobulin G (IgG) alloantibodies to FVIII.2-5 These alloantibodies, known as inhibitors, block the activity of FVIII, decreasing or even eliminating the effectiveness of factor replacement.2,6 As a result, FVIII inhibitors, which occur in ∼20% to 40% of patients with severe hemophilia A and 5% of patients with mild/moderate hemophilia A, render FVIII infusions ineffective. This, in turn, makes bleeding difficult to control and prevent, resulting in increased morbidity and mortality, increased cost of care, and decreased quality of life.7,8 The principal strategy currently available for inhibitor eradication is immune tolerance induction, which entails frequent and prolonged exposure to the FVIII protein in an effort to induce peripheral tolerance. Although successful in 60% to 70% of cases, this treatment continues to suffer from the significant time and considerable expense required for implementation,8-10 making strategies to avoid inhibitor formation altogether paramount to effective patient care. However, despite the significant clinical implications of inhibitor development, there are currently no prophylactic agents available for inhibitor prevention.
Previous studies suggest that a combination of genetic and environmental factors likely influence inhibitor development.11,12 However, the immune mechanisms underlying inhibitor formation are incompletely understood. CD4 T cells, in particular T follicular helper cells, work in concert with follicular B cells to drive germinal center reactions within B-cell follicles, which in turn generates B cells that produce high-affinity antibodies and B-cell memory.13,14 As a result, several studies have defined key aspects of the T- and B-cell response to FVIII, including dominant CD4 T-cell and B-cell FVIII epitopes.15-19 However, before a germinal center reaction can occur, antigenic substrate must be available. Recent studies suggest that the transport of antigen to the B-cell follicle is itself a highly regulated process that allows the immune system to discriminate antigenic material from self.20-23 In particular, within the blood compartment, cells within the marginal sinus of the spleen, which resides at the interface of the red and white pulp, appear to be uniquely poised to survey blood for this very purpose.24,25 Although a variety of cells facilitate this process, marginal zone (MZ) B cells represent an innate-like B-cell population and are the only cells in the MZ known to possess antigen-specific receptors by virtue of the unique recombination events that generate their B-cell receptors.20-23 As a result, although a variety of cells may engage FVIII within the MZ, MZ B cells may possess the unique capacity to specifically recognize and then respond to FVIII.
MZ B cells not only possess the ability to immediately generate antibody in response to antigenic challenge and then traffic that antigen to the B-cell follicle,21,26,27 but these cells can also facilitate antigen delivery to dendritic cells (DCs), which in turn can enhance CD4 T-cell activation.23 As MZ B cells have also been shown to directly drive CD4 T-cell activation,20 these cells appear to play a key role in orchestrating a wide variety of downstream immune events required for inhibitor formation.25,28-30 Consistent with the possible role of MZ B cells in this process, recent data suggest that the spleen, and, more particularly, MZ macrophages (MZMs) and other macrophage populations that reside in the MZ of the spleen are also important in the development of inhibitor formation.31 As MZMs and other marginal sinus constituents can trap foreign antigen and work in concert with MZ B cells to respond to blood-borne antigen,32-35 we tested the hypothesis that MZ B cells may be key early regulators of inhibitor formation following FVIII exposure and that targeting this population may therefore provide a unique strategy to prevent FVIII inhibitor development.
Methods
Mice and materials
FVIII knockout mice (hemophilia A mice, TKO) on a C57BL/6 background were used for all experiments.36 TKO mice possess a deletion of the entire F8 coding sequence36 ; no F8 messenger RNA is detectable in these mice. TKO mice and E16 hemophilia A mice exhibit a similar bleeding phenotype as measured by factor activity and a tail-snip assay, and develop similar anti-FVIII antibody titers following recombinant FVIII exposure. Eight- to 12-week-old male and female mice were used. All animals were housed and bred in cages at the Emory University Department of Animal Resources facilities, and all experiments were performed under animal protocols approved by the Institutional Animal Care and Use Committee of Emory University. Recombinant human FVIII was generated as outlined previously,37 with additional recombinant human FVIII generously donated by Hemophilia of Georgia.
Confocal microscopy
Hemophilia A mice received either 1, 2, 4, or 25 μg of recombinant human FVIII or saline delivered via retro-orbital injection. Fifteen minutes after FVIII administration, spleens were harvested, frozen in isopentane using TissueTek freezing medium (VWR Scientific, Randor, PA), and sectioned, followed by fixation, as previously described.31 The 7-μm-thick sections were incubated in 0.5% blocking buffer (phosphate-buffered saline [PBS] + 0.5% fetal bovine serum) for 2 hours at room temperature. Sections were then stained for 1 hour at room temperature with polyclonal sheep anti-human FVIII (HTI, Essex Junction, VT) diluted 1/100 in blocking buffer. After washing in PBS, fluorescein isothiocyanate (FITC) anti-sheep IgG secondary antibody (Sigma-Aldrich, St. Louis, MO) diluted 1/100 in blocking buffer was applied for 1 hour at room temperature. Sections were washed and then subsequently stained with phycoerythrin (PE)-CF594 rat anti-mouse B220, and PE rat anti-mouse CD1d (BD Biosciences, San Jose, CA) diluted 1/100 in blocking buffer for 1 hour at room temperature. Sections were again washed in PBS and mounted using Prolong Gold antifade mounting media (ThermoFisher Scientific, Waltham, MA). Images using 10× and 40× objectives were captured using the Leica SP8 multiphoton confocal microscope and analyzed using Leica application suite (LAS) Advance Fluorescence lite software.
Cellular depletion and FVIII administration
To achieve MZ B-cell depletion, hemophilia A mice received intraperitoneal (IP) injections of 100 μg of the MZ B-cell–depleting antibodies anti-CD11a (clone M17/4; Bioxcell, West Lebanon, NH) and anti-CD49d (clone PS/2; Bioxcell) in 400 μL of sterile saline on days −4, −2, 10, and 20 as outlined previously.38 Control mice received IP injections of 400 μL of sterile saline or 100 μg of each isotype control antibody (rat IgG2b [clone: LTF2] and rat IgG2a [clone 2A3]; Bioxcell) according to the same depletion schedule. Depletion was evaluated using flow cytometric analysis (described in the next section). Human recombinant FVIII (2 or 4 μg) in a 100-μL total volume of sterile saline was administered via retro-orbital injection according to the dosing and administration schedules outlined. Mice administered polyinosinic:polycytidylic acid (PIC) (Invivogen; San Diego, CA) received 100 μg via IP injection on days 0 and 35, 2 to 4 hours prior to FVIII injection.
Flow cytometry and plasma analysis for anti-FVIII antibodies
To verify MZ B-cell depletion, spleens were harvested from representative hemophilia A mice and 2 × 105 splenocytes were stained with Alexa 700 anti-B220, Pacific Blue anti-CD21, allophycocyanin anti-CD23, FITC anti-IgM, BV650 anti-IgD, PECy7 anti-CD5, PE anti-CD1d (BioLegend, San Diego, CA), and FITC anti-IgM antibodies (BD Biosciences) diluted 1/100 in fluorescence-activated cell sorting buffer for 30 minutes at 4°C as outlined previously.38 The percentage of MZ B cells (B220+CD21hiCD23lo/−) present in the spleen of depleted mice, isotype control, and saline-injected control mice was determined using an LSR-II flow cytometer (BD Biosciences), and data were analyzed using FlowJo software version 9.9.
To examine anti-FVIII inhibitor formation in MZ B-cell–depleted, isotype control–treated, or saline control–treated hemophilia A mice in the presence or absence of PIC, blood was collected from the orbital venous plexus with heparinized capillary tubes 7 days after the last injection of FVIII for all specified time points. An enzyme-linked immunosorbent assay (ELISA) and a modified Bethesda assay were used for measuring anti-FVIII IgG and FVIII inhibitor titers, respectively, as previously described.39-41
Statistical analysis
Significance testing was determined by a 1-way analysis of variance (ANOVA) test with a post hoc Tukey test using Prism 7.0 (GraphPad Software, La Jolla, CA). P values <.05 were considered statistically significant.
Results
FVIII localizes in the splenic marginal sinus and colocalizes with MZ B cells
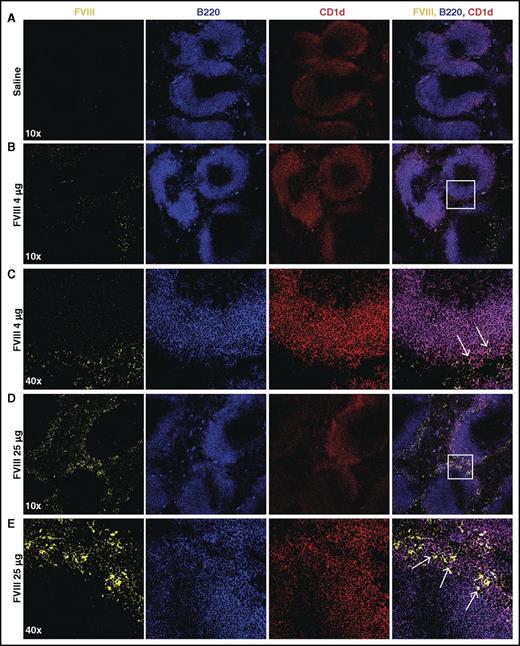
Given the possible role of the spleen and distinct macrophage populations that reside within the MZ of the spleen in the development of FVIII inhibitors,31 we first sought to determine whether FVIII also associates with other key immune players within the MZ, specifically MZ B cells. To accomplish this, we injected recombinant FVIII into hemophilia A mice at concentrations commonly used to induce inhibitors in this model system,31 followed by splenic harvest 15 minutes after injection and confocal examination for FVIII distribution. Injection of 1, 2, or 4 μg of FVIII resulted in detectable FVIII, which localized to the marginal sinus of the spleen and, at higher magnification, displayed colocalization with MZ B cells (Figure 1A-C; supplemental Figure 1, available on the Blood Web site), strongly suggesting possible MZ B-cell interactions with FVIII shortly after it enters the circulation. As FVIII was largely confined to the MZ following FVIII injection, we next sought to determine whether significantly higher doses of FVIII would result in FVIII localization within the B-cell follicle. Surprisingly, even at a supratherapeutic dose (25 μg), FVIII failed to penetrate the B-cell follicle and remained in the marginal sinus, where it again colocalized with MZ B cells (Figure 1D-E). Taken together, these results suggest that MZ B cells engage FVIII in the spleen, followed by initiation and subsequent orchestration of a FVIII immune response.
FVIII localizes in the marginal sinus of the spleen with MZ B cells. FVIII-deficient mice were injected with saline (A), 4 μg of FVIII (B-C), or 25 μg of FVIII (D-E), followed by splenic harvest 15 minutes post injection. Frozen spleen sections were stained with polyclonal sheep anti-FVIII-FITC (yellow), B220-PE-CF594 (blue), and CD1d-PE (red). Images were obtained using the Leica SP8 multiphoton confocal microscope and are shown at 10× and 40× magnification. White box indicates area magnified at 40×. Arrows indicate colocalization of FVIII and CD1d (MZ B cells).
FVIII localizes in the marginal sinus of the spleen with MZ B cells. FVIII-deficient mice were injected with saline (A), 4 μg of FVIII (B-C), or 25 μg of FVIII (D-E), followed by splenic harvest 15 minutes post injection. Frozen spleen sections were stained with polyclonal sheep anti-FVIII-FITC (yellow), B220-PE-CF594 (blue), and CD1d-PE (red). Images were obtained using the Leica SP8 multiphoton confocal microscope and are shown at 10× and 40× magnification. White box indicates area magnified at 40×. Arrows indicate colocalization of FVIII and CD1d (MZ B cells).
MZ B-cell depletion prevents FVIII inhibitor formation
To more specifically test the role of MZ B cells in FVIII antibody formation, we next developed a protocol to deplete MZ B cells from hemophilia A mice. Given that multiple doses of FVIII are required for the development of a measurable anti-FVIII antibody response in this model,36,42,43 and to mimic the administration schedule often used in hemophilia A patients, we sought to develop an approach that would enable MZ B-cell depletion throughout the first month of injection, thereby allowing for the first 4 weekly injections of FVIII to occur in the absence of MZ B cells. As MZ B cells only reside within the spleen in mice, parallel sampling of the spleen to determine MZ B-cell depletion efficacy and measurement of anti-FVIII antibody formation is not possible. Therefore, to develop a protocol to deplete MZ B cells for 1 month, we first depleted and examined hemophilia A mice over time. To accomplish this, MZ B cells were initially depleted by 2 doses of anti-CD11a and anti-CD49d antibodies, which are capable of specifically depleting MZ B cells,38,44 on days −4 and −2 prior to the first planned FVIII infusion. As previous studies suggest that MZ B cells can begin to repopulate the spleen between 10 and 14 days following initial depletion,44 mice received additional doses of both MZ B-cell–depleting antibodies on days 10 and 20 following the first initial planned FVIII infusion. To determine the efficacy of this approach, the percentage of splenic MZ B cells, defined as B220+CD21hiCD23lo/− cells,38 was examined at indicated time points following depletion. At each time point examined, recipients that received MZ B-cell–depleting antibodies displayed >99% depletion when compared with control mice (Figure 2).
Depletion of MZ B cells in mice with hemophilia A. Where indicated, mice with hemophilia A received MZ B-cell–depleting antibodies at days −4, −2, +10, and +20. Splenocytes from representative mice were then evaluated by flow cytometry to determine successful depletion. Representative flow plots of B220+CD21hiCD23lo/− cells following injection of saline, MZ B-cell–depleting antibody (MZ B cell depl), or isotype control antibody at days 0 (A), +10 (C), and +20 (E). Quantitative analysis of B220+CD21hiCD23lo/− cells (% MZ B cells) following administration of saline, MZ B-cell–depleting antibody (MZ B cell depl), or isotype control antibody at days 0 (B), +10 (D), and +20 (F). Error bars indicate standard deviation (SD). Horizontal lines represent mean of each group. *P < .03, **P < .003, ****P < .0001, 1-way ANOVA, post hoc Tukey test. SSC, side scatter.
Depletion of MZ B cells in mice with hemophilia A. Where indicated, mice with hemophilia A received MZ B-cell–depleting antibodies at days −4, −2, +10, and +20. Splenocytes from representative mice were then evaluated by flow cytometry to determine successful depletion. Representative flow plots of B220+CD21hiCD23lo/− cells following injection of saline, MZ B-cell–depleting antibody (MZ B cell depl), or isotype control antibody at days 0 (A), +10 (C), and +20 (E). Quantitative analysis of B220+CD21hiCD23lo/− cells (% MZ B cells) following administration of saline, MZ B-cell–depleting antibody (MZ B cell depl), or isotype control antibody at days 0 (B), +10 (D), and +20 (F). Error bars indicate standard deviation (SD). Horizontal lines represent mean of each group. *P < .03, **P < .003, ****P < .0001, 1-way ANOVA, post hoc Tukey test. SSC, side scatter.
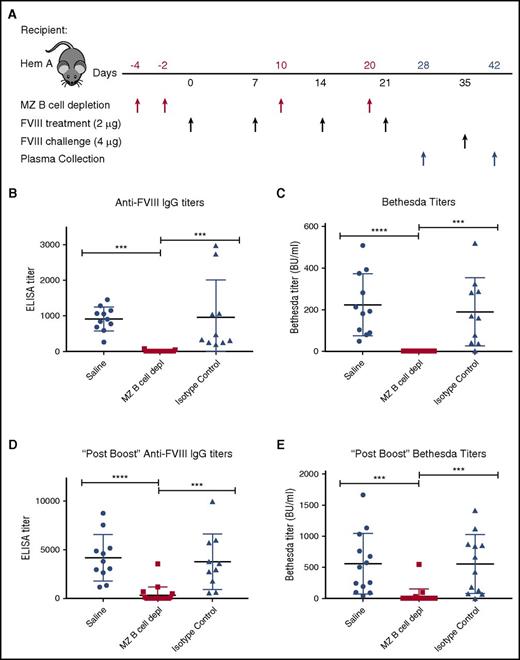
Having developed an approach to deplete MZ B cells, we next defined the impact of MZ B-cell depletion on the FVIII immune response. As outlined in Figure 3A, hemophilia A mice first underwent MZ B-cell depletion, followed by 4 weekly injections of 2 μg of recombinant FVIII. Control mice received either an injection of saline or isotype control antibodies in parallel, followed by identical FVIII administration schedules. To evaluate the impact of MZ B-cell depletion on anti-FVIII antibody formation, an ELISA was performed on plasma taken 7 days after the last FVIII injection. Although no anti-FVIII antibodies could be detected in hemophilia A mice at baseline (data not shown), saline-injected and isotype control–treated mice generated anti-FVIII antibodies following FVIII exposure (Figure 3B). In contrast, mice depleted of MZ B cells did not produce detectable anti-FVIII antibodies (Figure 3B). The Bethesda assay was then performed to determine the inhibitory activity of any anti-FVIII antibodies that may be present. As predicted by the lack of detectable anti-FVIII antibodies in MZ B-cell–depleted recipients as measured by ELISA, removal of MZ B cells prevented FVIII inhibitor formation (Figure 3C). Following the initial round of FVIII administration, all mice received an additional double-dose “boost” of 4 μg of FVIII, as done previously.36 Despite this higher dose exposure of FVIII, the majority of recipients depleted of MZ B cells during the primary exposure experienced a greatly reduced response. Indeed, the majority of MZ B-cell–depleted recipients completely failed to develop anti-FVIII antibodies (68%) or FVIII inhibitors (90%) when examined 7 days after administration of a “boosted” dose of FVIII (Figure 3D-E), indicating variable MZ B-cell reconstitution at the time of FVIII boost.
MZ B-cell depletion prevents FVIII inhibitor development. (A) Mice with hemophilia A (Hem A) received MZ B-cell–depleting antibodies (MZ B cell depl), isotype control antibody, or saline at the indicated time points (red arrows), followed by FVIII at the specified dose (black arrows). After 4 weekly doses of 2 μg of FVIII, day 28 plasma was evaluated for anti-FVIII IgG by ELISA (B) and inhibitor titer by Bethesda assay (C). After an additional “boost” dose of 4 μg of FVIII on day 35, day 42 plasma was evaluated for anti-FVIII IgG by ELISA (D) and inhibitor titer by Bethesda assay (E). Error bars indicate SD. Horizontal lines represent mean of each group. ***P < .0002, ****P < .0001, 1-way ANOVA, post hoc Tukey test.
MZ B-cell depletion prevents FVIII inhibitor development. (A) Mice with hemophilia A (Hem A) received MZ B-cell–depleting antibodies (MZ B cell depl), isotype control antibody, or saline at the indicated time points (red arrows), followed by FVIII at the specified dose (black arrows). After 4 weekly doses of 2 μg of FVIII, day 28 plasma was evaluated for anti-FVIII IgG by ELISA (B) and inhibitor titer by Bethesda assay (C). After an additional “boost” dose of 4 μg of FVIII on day 35, day 42 plasma was evaluated for anti-FVIII IgG by ELISA (D) and inhibitor titer by Bethesda assay (E). Error bars indicate SD. Horizontal lines represent mean of each group. ***P < .0002, ****P < .0001, 1-way ANOVA, post hoc Tukey test.
FVIII rechallenge after MZ B-cell reconstitution results in a primary immune response
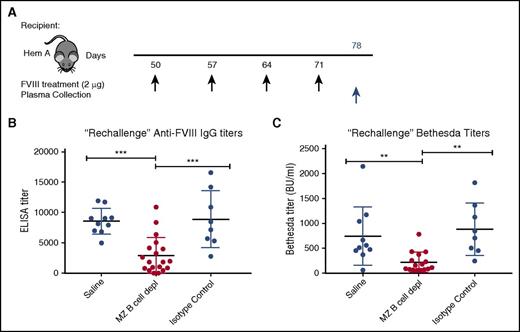
Although the attenuated response following FVIII boost administration in recipients initially depleted of MZ B cells may reflect incomplete MZ B-cell reconstitution, it is also possible that MZ B-cell removal facilitates the development of tolerance following FVIII exposure. To determine whether MZ B-cell–depleted recipients exposed to FVIII were tolerized, we allowed MZ B-cell reconstitution to occur prior to a second round of FVIII exposure. As it is not possible to sample spleens and examine MZ B-cell reconstitution in FVIII-exposed hemophilia A mice, control and experimental hemophilia A mice were “rechallenged” with 4 additional weekly doses of 2 μg of FVIII 30 days following the last MZ B-cell depletion (Figure 4A), when complete MZ B-cell reconstitution is most likely.44 Plasma was then harvested 7 days following the last FVIII injection and the development of anti-FVIII antibodies was evaluated by ELISA and Bethesda assay. After FVIII rechallenge, mice that previously underwent MZ B-cell depletion developed anti-FVIII antibodies and inhibitors at titers that were very similar to the initial titers developed by control mice after the first 4 injections (Figure 4B-C), consistent with a primary immune response. In contrast, the titers in hemophilia A mice with no prior MZ B-cell depletion exhibited a significant increase in titers after the second round of FVIII injections (cf. Figure 3D vs Figure 4B and Figure 3E vs Figure 4C). Together, these data suggest that MZ B-cell depletion likely prevents an initial antibody response following FVIII exposure, but our data do not rule out a role of MZ B cells in immunological tolerance.
Rechallenge with FVIII after MZ B-cell repopulation results in primary immune response. (A) Mice with hemophilia A that previously underwent MZ B-cell depletion (MZ B cell depl) with FVIII challenge, received a “rechallenge” with 2 μg of FVIII at the indicated time points (black arrows), followed by plasma collection 1 week after the last injection (blue arrow). Plasma was evaluated for anti-FVIII IgG by ELISA (B) and inhibitor titer by Bethesda assay (C). Error bars indicate SD. Horizontal lines represent mean of each group. **P < .003, ***P < .0002, 1-way ANOVA, post hoc Tukey test.
Rechallenge with FVIII after MZ B-cell repopulation results in primary immune response. (A) Mice with hemophilia A that previously underwent MZ B-cell depletion (MZ B cell depl) with FVIII challenge, received a “rechallenge” with 2 μg of FVIII at the indicated time points (black arrows), followed by plasma collection 1 week after the last injection (blue arrow). Plasma was evaluated for anti-FVIII IgG by ELISA (B) and inhibitor titer by Bethesda assay (C). Error bars indicate SD. Horizontal lines represent mean of each group. **P < .003, ***P < .0002, 1-way ANOVA, post hoc Tukey test.
Depletion of MZ B cells prevents FVIII inhibitor formation in an inflamed state
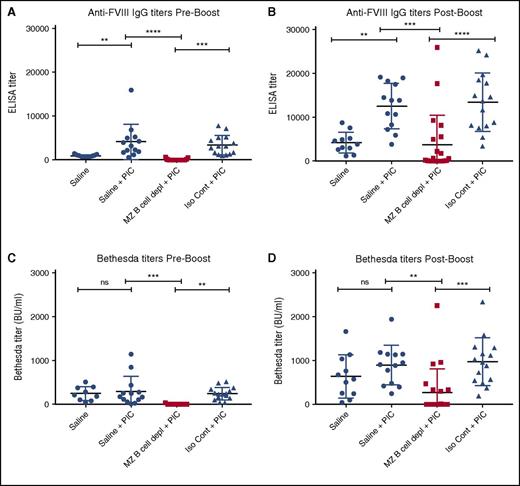
Previous studies suggest that recipient inflammation during the time of FVIII administration may enhance the immune response to FVIII.12 Recent studies also suggest that viral-like inflammation in particular can directly facilitate DC uptake of blood-borne antigens and subsequent activation of CD4 T cells,45 suggesting that, under certain circumstances, an inflammatory immune state may bypass the MZ B-cell requirement for FVIII inhibitor formation. As a result, we next examined whether viral-like inflammation alters FVIII inhibitor formation and whether MZ B-cell depletion continues to prevent anti-FVIII antibody development in this setting. To accomplish this, hemophilia A mice underwent MZ B-cell depletion, followed by administration of PIC, an immune adjuvant known to induce a viral-like inflammatory response,46,47 at the time of the first of 4 weekly injections of FVIII, as done previously.47 The ELISA and the Bethesda assay were then performed to evaluate the immune response to FVIII. Hemophilia A mice exposed to FVIII in the presence of PIC displayed higher titers of FVIII antibodies than similar recipients that received FVIII following saline injection alone (Figure 5A,C), strongly suggesting that underlying recipient inflammation may impact the magnitude of antibody formation. Importantly, although PIC enhanced anti-FVIII production in nondepleted control recipients, MZ B-cell depletion continued to completely prevent anti-FVIII antibody development even in the presence of PIC (Figure 5A,C), with MZ B-cell–depleted recipients that received a “boost” dose likewise experiencing a severely blunted response compared with controls (Figure 5B,D). These results strongly suggest that MZ B cells appear to serve as key regulators of FVIII inhibitor formation even under conditions of recipient inflammation.
MZ B-cell depletion prevents FVIII inhibitor development when administered with PIC. Mice with hemophilia A received either MZ B-cell–depleting antibodies (MZ B cell depl), saline, or isotype control (Iso Cont) antibodies, followed by injection of PIC and 4 weekly doses of 2 μg of FVIII. Control mice that received saline followed by FVIII without PIC are included for comparison. Plasma was collected and evaluated for anti-FVIII IgG by ELISA (A). Plasma was also evaluated for anti-FVIII IgG by ELISA after an additional “boost” dose of 4 μg of FVIII (B). Plasma was evaluated for inhibitor titer by Bethesda assay both before (C) and after (D) the “boost” dose of FVIII. Error bars indicate SD. Horizontal lines represent mean of each group. **P < .003, ***P < .0002, ****P < .0001, 1-way ANOVA, post hoc Tukey test. ns, not significant.
MZ B-cell depletion prevents FVIII inhibitor development when administered with PIC. Mice with hemophilia A received either MZ B-cell–depleting antibodies (MZ B cell depl), saline, or isotype control (Iso Cont) antibodies, followed by injection of PIC and 4 weekly doses of 2 μg of FVIII. Control mice that received saline followed by FVIII without PIC are included for comparison. Plasma was collected and evaluated for anti-FVIII IgG by ELISA (A). Plasma was also evaluated for anti-FVIII IgG by ELISA after an additional “boost” dose of 4 μg of FVIII (B). Plasma was evaluated for inhibitor titer by Bethesda assay both before (C) and after (D) the “boost” dose of FVIII. Error bars indicate SD. Horizontal lines represent mean of each group. **P < .003, ***P < .0002, ****P < .0001, 1-way ANOVA, post hoc Tukey test. ns, not significant.
Discussion
FVIII inhibitors can significantly complicate hemophilia A patient management. However, despite the often-severe clinical impact of FVIII inhibitors,2,6-8 there are no prophylactic therapies to prevent their formation. This is in part due to a lack of understanding regarding the key initiating immune mechanisms underlying their development, and therefore the absence of a precise target for prophylactic agents. Thus, identification of specific cells involved in the immune response is pivotal to the development of novel strategies to prevent inhibitor formation in patients with hemophilia A. The ability of MZ B-cell depletion to prevent FVIII inhibitor formation in both noninflamed and inflamed recipients, coupled with the proposed roles of MZ B cells in orchestrating key downstream immune events following antigen exposure, strongly suggests that MZ B cells may not only serve as critical initiators in the immune response to FVIII, but that these cells may also serve as key targets in preventing inhibitor development in patients with hemophilia A.
The spleen is a highly organized blood-filtration system that plays a primary role in the development of immunity to many blood-borne antigens. As an immune response to antigens is spatially and temporally regulated in highly specialized secondary lymphoid compartments, appropriate delivery of antigens into the B-cell follicle and other key destinations within the white pulp can serve as a regulatory checkpoint in the development of an immune response to a blood-borne antigen.28,48,49 As MZ B cells serve as a specialized innate-like B-cell subset with the ability to react with antigen-specific cargo secondary to distinct B-cell receptors,29,50 MZ B cells are uniquely poised to specifically recognize and respond to FVIII following exposure. Given previous results that demonstrated that macrophages within the MZ of the spleen are also likely important in regulating FVIII inhibitor development,31 the results of the present study suggest that MZ B cells likely work in concert with MZMs to recognize and respond to FVIII following exposure. Consistent with this, previous studies demonstrate that MZMs, MZ B cells, and other populations within the marginal sinus work in concert to orchestrate the initial recognition and response to blood-borne antigens.32-35 MZ B cells may not only facilitate early anti-FVIII antibody production but, given previous results demonstrating a requirement of follicular B cells and CD4 T cells in the development of anti-FVIII antibodies,15-19,51 MZ B cells may also facilitate FVIII trafficking to the B-cell follicle as well as DCs and CD4 T cells.20,28,29,38,50,52,53 In this way, MZ B cells may play a key role in initiating key downstream immune events required for anti-FVIII antibody formation. As a recent study suggested that MZ B cells may also be a target of FVIII tolerance,51 induction of tolerance may reflect alterations in the ability of MZ B cells to engage or respond to FVIII following exposure, which would be predicted to impact the potential activation of subsequent downstream immune players. Use of previous models, such as hemophilia A mice that express human FVIII,54,55 may be useful in defining the role of MZ B cells in the development of FVIII tolerance.
Not all patients with hemophilia A form antibodies following FVIII exposure.2,56-58 Although major histocompatibility complex restriction and various immunological polymorphisms have been demonstrated to play roles in dictating immune responses to FVIII or FIX,15,16,59 inherited factors alone fail to definitively predict inhibitor formation. Recent studies have demonstrated that the precursor frequency of antigen-specific T cells can significantly influence the immunological outcome of antigen exposure.60-62 Although the potential impact of antigen-specific MZ B cells in dictating immune responsiveness has not been formally evaluated, as MZ B cells do possess a restricted and distinct repertoire of antibody specificities that can differ between individuals,23,60-67 differences in the precursor frequency of FVIII-specific MZ B cells may also impact the likelihood of anti-FVIII antibody formation. Therefore, in addition to the potential impact of inherited genetic modifiers,11,59 differences in the frequency of FVIII-specific MZ B cells may represent an additional contributing factor that influences the likelihood of inhibitor formation following FVIII exposure.
Unlike microbes, FVIII possesses no known canonical innate immune ligands capable of alerting the immune system of impending danger. The lack of discernable immune activators, coupled with the putative need for danger signals to stimulate robust immunity,68 suggests that the inflammatory state of a recipient may significantly impact a FVIII immune response. Consistent with this, previous studies have correlated recipient inflammation at the time of FVIII administration with inhibitor development.12 Conversely, a recent study demonstrated that vaccination in hemophilia A mice at the time of FVIII administration not only failed to enhance FVIII inhibitor formation, but also actually inhibited anti-FVIII alloantibody development.69 In contrast to the present study, in which PIC was administered in the absence of antigen, FVIII administration in the setting of vaccination appears to cause competition for antigenic substrate, possibly deviating the immune response away from FVIII.69 However, vaccination and PIC administration may result in localized vs systemic inflammatory responses, respectively. Thus, the generalized nature of PIC-induced inflammation may also contribute to the impact of PIC on anti-FVIII antibody formation. Therefore, although MZ B cells appear to be critical when exposing hemophilia A mice to FVIII IV, alternative routes of administration in the setting of inflammation may result in the engagement of alternative immune populations. However, MZ B cells exist in a partially activated state and require a lower activation threshold under noninflammatory conditions. Thus, the ability of MZ B cells to facilitate inhibitor formation following intravascular delivery may account for the ability of individuals to respond to FVIII and other therapeutics following IV administration in the absence of a known adjuvant.70-73
Although MZ B cells are restricted to the spleen in mice, in humans, MZ B cells are found in the spleen,24,25 other secondary lymphoid organs, and in circulation.25,28-30 However, given that MZ B cells are defined in each setting by their MZ location as well as their ability to trap, traffic, and rapidly respond to antigen in mice and humans,23,25,28-30 we predict that MZ B cells will play a similar role in patients with hemophilia A. Thus, targeting MZ B cells may provide a useful approach to prevent inhibitor formation. However, the specificity of MZ B-cell depletion in humans and potential unwanted infectious risks while the MZ B cells repopulate remain important considerations. Therefore, approaches designed to more transiently target MZ B-cell function may reduce the risks associated with infection. Furthermore, as recipient inflammation enhanced inhibitor formation in hemophilia A mice, administration of a prophylactic agent that targets MZ B cells during episodes of planned inflammation, such as scheduled surgical procedures, when FVIII is also required, may be helpful in preventing inhibitor formation. In contrast, other therapies that have been suggested for the prevention or eradication of inhibitors, such as rituximab,74,75 often result in a longer-lasting effect, with coincident increased risk for infectious complications.
In summary, we have demonstrated that FVIII localizes to the marginal sinus of the spleen and colocalizes with MZ B cells. Furthermore, depletion of this specific B-cell population prevents anti-FVIII antibody formation after exposure to FVIII in mice with hemophilia A both in the baseline and inflamed states, suggesting that MZ B cells are an important initiating step in inhibitor formation. MZ B cells may therefore serve as a potential therapeutic target to prevent inhibitor formation in patients with hemophilia A.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Hemophilia of Georgia (S.R.S., P.L., and S.L.M.), the Burroughs Wellcome Trust Career Award to Medical Scientists (S.R.S.), and National Institutes of Health, National Heart, Lung, and Blood Institute grant U54 HL112309 (P.L. and S.L.M.).
Authorship
Contribution: P.E.Z., C.C., W.H.B., S.R.P., and C.M.A. conducted the experiments, while also helping with experimental design overseen by P.L., S.L.M., and S.R.S.; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sean R. Stowell, Center for Transfusion Medicine and Cellular Therapies, Department of Laboratory Medicine and Pathology, Emory University School of Medicine, 615 Michael St, Atlanta, GA 30322; e-mail: srstowe@emory.edu; or Shannon L. Meeks, Aflac Cancer and Blood Disorders Center, Emory University/Children’s Healthcare of Atlanta, 2015 Uppergate Dr #442, Atlanta, GA 30322; e-mail: shannon.meeks@choa.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal