To the editor:
Cell surface markers of adult hematopoietic stem cells (HSCs) are well established, but fewer markers are available for embryonic HSCs and their immediate precursors (pre-HSCs). HSCs differentiate from hemogenic endothelial cells in the major arteries of the embryo [dorsal aorta, umbilical, vitelline (A+U+V)]. They briefly reside in hematopoietic clusters attached to the arterial wall, then are released into the circulation to colonize the fetal liver (FL). All of the >500 hematopoietic cluster cells (HCCs) express Kit, a marker of FL and bone marrow (BM) HSCs, and endothelial markers, such as vascular endothelial cadherin (CD144) and CD31.1 In mouse embryos, the few (1-3) adult repopulating HSCs within the clusters are Kit+, CD31+, CD144+, CD45+, CD43+, CD41low, CD201high, and Ly6a-GFP+ and lack expression of the BM and FL HSC markers Sca1 and CD150.2-7 Clusters also contain ∼65 immature HSCs called pre-HSCs that cannot engraft adult recipients directly, but can be matured ex vivo into HSCs that can engraft.8 Pre-HSCs [and less mature precursors (pro-HSCs)] are CD144+CD41+Kit+ and can be subdivided based on CD43 and CD45 expression into (from least to most mature) pro-HSCs (CD43–CD45–), type I pre-HSCs (CD43+CD45–), and type II pre-HSCs (CD43+CD45+).6,9 Also in the major arteries are ∼500 to 1000 progenitors with lymphoid potential, which share markers with type II pre-HSCs/HSCs.10,11
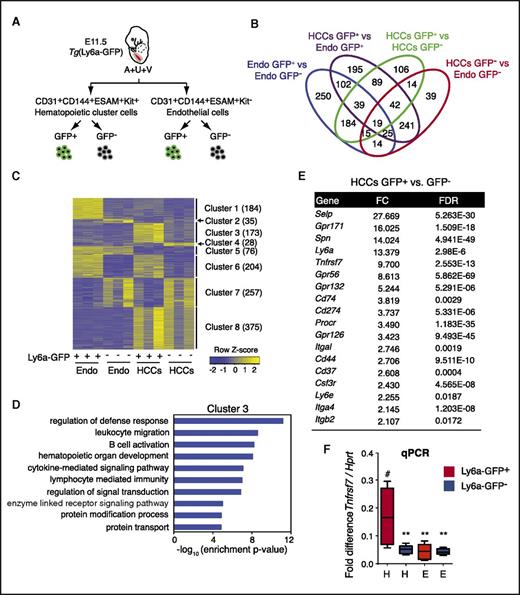
The transgene Ly6a-GFP marks all progenitors with lymphoid potential, pre-HSCs, and HSCs within HCCs in the major arteries (A+U+V)11,12 (Marijke Maijenburg, Joanna Tober, et al, unpublished data), and HSCs differentiate from Ly6a-GFP+ endothelial cells in vivo.13 To identify novel cell surface markers that could be used in lieu of the Ly6a-GFP transgene to further enrich pre-HSCs and HSCs, we determined the gene expression profile of embryonic day (E) 11.5 Ly6a-GFP+ HCCs (CD31+CD144+ESAM+Kit+) by RNA sequencing (GEO accession number GSE97509), and compared it to profiles of Ly6a-GFP- HCCs, Ly6a-GFP+ endothelial cells (CD31+CD144+ESAM+Kit–), and Ly6a-GFP– endothelial cells (Figure 1A-B; supplemental Table 1, available on the Blood Web site). A total of 1380 genes were differentially expressed across the 4 populations. We performed consensus clustering of these genes (Figure 1C). Cluster 3, which consists of 173 genes with higher expression in Ly6a-GFP+ HCCs compared with the other 3 populations, generated enriched gene ontology terms associated with leukocyte functions, such as “regulation of defense response,” “leukocyte migration,” and “B-cell activation” (Figure 1C-D; supplemental Table 2). Among cluster 3 genes were 14 encoding cell surface proteins, including known markers of embryonic HSCs, such as Spn (encoding CD43)6 and Gpr5614 (Figure 1E).
RNA sequence analysis to identify markers of pre-HSCs and embryonic HSCs. (A) Schematic showing the 4 cell populations that were compared. (B) Venn diagram of differentially expressed genes (DEGs) from pairwise comparisons. DEGs were identified by using a false discovery rate (FDR) cutoff <0.05 and a fold change cutoff >2. (C) Expression clusters defined by using consensus clustering and DEGs. Color indicates row-wise normalized expression levels. Numbers in parentheses indicate the number of genes in each cluster. (D) Gene ontology (GO) terms associated with genes in cluster 3. Level 5 biological process terms were used for enrichment analysis. Enrichment P values were computed by using the hypergeometric distribution. Nominal P values were corrected for multiple testing by using the Benjamini-Hochberg method. (E) List of cell surface marker genes in cluster 3 with more than twofold changes in gene expression in Ly6a-GFP+ HCCs relative to Ly6a-GFP– HCCs or Ly6a-GFP+/− endothelial cells. (F) Quantitative polymerase chain reaction analysis of Tnfrsf7 messenger RNA in all 4 populations. n = 6, whiskers represent the 5th to 95th percentile. Significance is by 1-way analysis of variance and Dunnett’s multiple comparison test with Ly6a-GFP+ HCCs as a comparator (#). **P < .01. E, endothelial cells; Endo, endothelial cells (CD31+CD144+ESAM+Kit–); FC, fold change; H, hematopoietic clusters.
RNA sequence analysis to identify markers of pre-HSCs and embryonic HSCs. (A) Schematic showing the 4 cell populations that were compared. (B) Venn diagram of differentially expressed genes (DEGs) from pairwise comparisons. DEGs were identified by using a false discovery rate (FDR) cutoff <0.05 and a fold change cutoff >2. (C) Expression clusters defined by using consensus clustering and DEGs. Color indicates row-wise normalized expression levels. Numbers in parentheses indicate the number of genes in each cluster. (D) Gene ontology (GO) terms associated with genes in cluster 3. Level 5 biological process terms were used for enrichment analysis. Enrichment P values were computed by using the hypergeometric distribution. Nominal P values were corrected for multiple testing by using the Benjamini-Hochberg method. (E) List of cell surface marker genes in cluster 3 with more than twofold changes in gene expression in Ly6a-GFP+ HCCs relative to Ly6a-GFP– HCCs or Ly6a-GFP+/− endothelial cells. (F) Quantitative polymerase chain reaction analysis of Tnfrsf7 messenger RNA in all 4 populations. n = 6, whiskers represent the 5th to 95th percentile. Significance is by 1-way analysis of variance and Dunnett’s multiple comparison test with Ly6a-GFP+ HCCs as a comparator (#). **P < .01. E, endothelial cells; Endo, endothelial cells (CD31+CD144+ESAM+Kit–); FC, fold change; H, hematopoietic clusters.
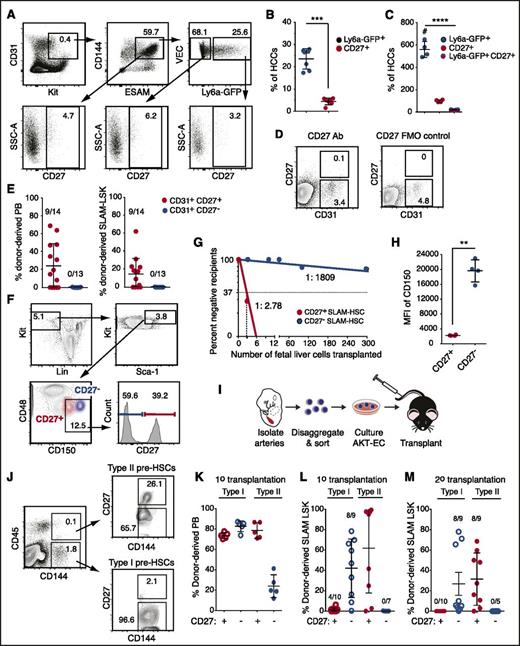
To identify new markers of embryonic HSCs and pre-HSCs, we analyzed Tnfrsf7, a gene in cluster 3 that encodes a BM HSC marker, CD27.15,16 CD27 is a proinflammatory member of the tumor necrosis factor receptor superfamily that plays a costimulatory role in T-cell receptor–mediated immune responses17,18 when activated by its ligand CD70. CD27 is expressed on lymphocytes, dendritic cells, on BM HSCs (in mice), and in humans on acute myelogenous leukemia (AML) blast cells.15,16,18-20 CD27 signals through the canonical and noncanonical NF-κB pathways and the JNK pathway through TRAF family adaptors in lymphocytes and activates Wnt signaling in AML cells.19-21 Tnfrsf7 transcripts were ∼10-fold higher in Ly6a-GFP+ HCCs than in the other 3 populations (Figure 1E-F), although CD27 protein was detectable on only 3.2% of the cells (Figure 2A-C). CD27 marked fewer HCCs than Ly6a-GFP; per embryo ∼560 HCCs were Ly6a-GFP+, 98 were CD27+, and 18 were Ly6a-GFP+CD27+ (Figure 2C). We fractionated CD31+ cells from the A+U+V based on CD27 expression and transplanted them into adult mice (Figure 2D-E). All HSCs in the A+U+V were in the CD31+CD27+ population (Figure 2E). Approximately 40% of cells in the CD48–CD150+lineage–Sca1+Kit+ (SLAM LSK) E14.5 FL population were CD27+ and contained most of the functional HSCs (Figure 2F-G). Back gating revealed that the CD27+ SLAM LSK cells were CD150medium and clearly distinct from the CD27– fraction, which was CD150high (Figure 2F,H). Erythromyeloid progenitors (EMPs) emerge before HSCs22 ; the majority of CD45+ EMPs in the E11.5 yolk sac and FL were CD27– (supplemental Figure 1A-C). In contrast, progenitors with lymphoid potential in the A+U+V, which emerge before HSCs,22 were enriched in the CD27+ population at both E10.5 and E11.5 (supplemental Figure 1D-G), similar to the enrichment we reported for Ly6a-GFP.11 In summary, CD27 marks all embryonic and FL HSCs, enriches for lymphoid progenitors in the major arteries, but is not expressed on most yolk sac–derived EMPs.
Embryonic HSCs in the major arteries, FL HSCs, and type II pre-HSCs are CD27+. (A) Representative scatter plots of CD27 expression on HCCs (CD31+Kit+CD144+ESAM+) and on the Ly6a-GFP+ and Ly6a-GFP– fractions of HCCs. Data are from the A+U+V of E11.5 Tg(Ly6a-GFP) embryos. (B) The average percentage (± SD) of Ly6a-GFP+ cells and Ly6a-GFP+CD27+ cells in the CD31+CD144+ESAM+Kit+ hematopoietic clusters (HCCs) (n = 7). Unpaired 2-tailed Student t test. ***P < .001. (C) The average number of cells in hematopoietic clusters with the indicated markers per embryo (mean ± SD, analysis of variance, Tukey’s multiple comparison test). ****P < .0001. (D) Representative sort plots for CD31+CD27+ and CD31+CD27– cells used in transplantation assays. Cells were sorted from the A+U+V of E11.5 embryos. (E) Left, average percentage (± SD) of donor-derived cells in the recipient PB 16 weeks posttransplantation. The number of repopulated animals/total number of recipients is indicated above each column. Right, average contribution to BM SLAM (CD48– CD150+) LSK cells. (F) Representative scatter plots of FL SLAM LSK cells from E14.5 fetuses separated based on CD27 expression. Lineage, Ter119, CD3e, B220, Gr1. Bottom left scatter plot shows the location of CD27+ and CD27– cells within the SLAM LSK population. (G) Limiting dilution transplantation of CD27+ and CD27– SLAM LSK cells. Reconstitution (>1% donor-derived BM SLAM LSK cells) was assessed at 16 weeks posttransplantation (n = 4-8 recipients per dose). The frequencies of HSCs were determined by extreme limiting dilution analysis.25 P = 2.9e-32. (H) Mean fluorescence intensity (MFI) of CD150 in the CD27+ and CD27– populations of SLAM LSK cells (n = 4). Unpaired 2-tailed Student t test. **P < .01. (I) Schematic for assessment of pre-HSCs. A+U+V were isolated, dissociated, cells sorted, cultured on Akt-EC for 7 days, and transplanted into irradiated recipients. (J) Representative sort plots of CD27+ and CD27– type I and II pre-HSCs. (K) The average percentage (± SD) of donor-derived PB cells 16 weeks posttransplantation into primary recipient mice. (L) Percentage of donor-derived SLAM LSK cells in recipient BM 16 weeks posttransplantation. (M) Percentage of donor-derived SLAM LSK cells in the BM of secondary recipients. A total of 500 000 BM cells from each primary recipient were injected into secondary recipients. FMO, fluorescence minus 1 control for CD27.
Embryonic HSCs in the major arteries, FL HSCs, and type II pre-HSCs are CD27+. (A) Representative scatter plots of CD27 expression on HCCs (CD31+Kit+CD144+ESAM+) and on the Ly6a-GFP+ and Ly6a-GFP– fractions of HCCs. Data are from the A+U+V of E11.5 Tg(Ly6a-GFP) embryos. (B) The average percentage (± SD) of Ly6a-GFP+ cells and Ly6a-GFP+CD27+ cells in the CD31+CD144+ESAM+Kit+ hematopoietic clusters (HCCs) (n = 7). Unpaired 2-tailed Student t test. ***P < .001. (C) The average number of cells in hematopoietic clusters with the indicated markers per embryo (mean ± SD, analysis of variance, Tukey’s multiple comparison test). ****P < .0001. (D) Representative sort plots for CD31+CD27+ and CD31+CD27– cells used in transplantation assays. Cells were sorted from the A+U+V of E11.5 embryos. (E) Left, average percentage (± SD) of donor-derived cells in the recipient PB 16 weeks posttransplantation. The number of repopulated animals/total number of recipients is indicated above each column. Right, average contribution to BM SLAM (CD48– CD150+) LSK cells. (F) Representative scatter plots of FL SLAM LSK cells from E14.5 fetuses separated based on CD27 expression. Lineage, Ter119, CD3e, B220, Gr1. Bottom left scatter plot shows the location of CD27+ and CD27– cells within the SLAM LSK population. (G) Limiting dilution transplantation of CD27+ and CD27– SLAM LSK cells. Reconstitution (>1% donor-derived BM SLAM LSK cells) was assessed at 16 weeks posttransplantation (n = 4-8 recipients per dose). The frequencies of HSCs were determined by extreme limiting dilution analysis.25 P = 2.9e-32. (H) Mean fluorescence intensity (MFI) of CD150 in the CD27+ and CD27– populations of SLAM LSK cells (n = 4). Unpaired 2-tailed Student t test. **P < .01. (I) Schematic for assessment of pre-HSCs. A+U+V were isolated, dissociated, cells sorted, cultured on Akt-EC for 7 days, and transplanted into irradiated recipients. (J) Representative sort plots of CD27+ and CD27– type I and II pre-HSCs. (K) The average percentage (± SD) of donor-derived PB cells 16 weeks posttransplantation into primary recipient mice. (L) Percentage of donor-derived SLAM LSK cells in recipient BM 16 weeks posttransplantation. (M) Percentage of donor-derived SLAM LSK cells in the BM of secondary recipients. A total of 500 000 BM cells from each primary recipient were injected into secondary recipients. FMO, fluorescence minus 1 control for CD27.
Pre-HSCs can be divided into type I and type II based on CD45 expression; type I pre-HSCs are CD144+CD45–, whereas more mature type II pre-HSCs are CD144+CD45+. We fractionated type I and type II pre-HSCs based on CD27 expression and cultured them on endothelial cells transduced with a lentivirus expressing myristoylated AKT (AKT-EC), which support the maturation of pre-HSCs into HSCs23,24 (Figure 2I-J). Cells derived from the cultures were then transplanted into adult mice. Both CD27+ and CD27– type I pre-HSCs contributed to the peripheral blood (PB) of primary transplant recipients (Figure 2K), with contribution biased toward lymphoid lineages (supplemental Figure 2). HSCs matured from CD27+ and CD27– type I pre-HSCs also contributed to BM SLAM LSK cells in primary transplant recipients. However, CD27+ type I pre-HSCs contributed to <10% of BM SLAM LSK cells and only engrafted 4 out of 10 recipients (Figure 2L). Furthermore, donor BM from primary transplant recipients did not engraft secondary recipients (Figure 2M), indicating that CD27 does not mark type I pre-HSCs. In contrast, CD27+ type II pre-HSCs matured into HSCs that contributed to BM SLAM LSK cells in both primary and secondary transplant recipients (Figure 2L-M). In summary, CD27 is expressed on type II but not type I pre-HSCs.
In this study, we show that CD27 expression is upregulated in a subset of HCCs, and CD27 marks HSCs, type II pre-HSCs, and progenitors with lymphoid potential within the cluster cells. Whether CD27 is simply a marker of type II pre-HSCs and embryonic HSCs, or whether it plays a functional role remains to be determined. Activation of CD27 on BM hematopoietic stem and progenitor cells by CD70 inhibits their differentiation and increases their number.18 Similarly, activation of CD27 on human AML blasts inhibited differentiation and promoted self-renewal by increasing symmetric cell divisions.19 Therefore, a possible role for CD27:CD70 signaling in the embryo may be to retain newly emerging type II pre-HSCs/HSCs in an undifferentiated state. Regardless, the demonstration that CD27 marks type II pre-HSCs and embryonic HSCs should provide an additional tool for identifying and studying these rare cells in the murine embryo.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE97509).
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant R01HL091724 (N.A.S.); NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R21 HD081054 (N.A.S. and K.T.); NIH, National Institute of General Medical Sciences grants R01 GM104369 (K.T.) and R01 GM108716 (K.T.); NIH, National Human Genome Research Institute grant R01 HG006130 (K.T.); and the Leukemia and Lymphoma Society (postdoctoral fellowship grant 0059-13) (Y.L.).
Contribution: Y.L., L.G., K.T., and N.A.S. designed the research and analyzed results; Y.L. and L.G. performed experiments; B.H. provided the AKT-ECs; and Y.L., K.T., and N.A.S. wrote the manuscript.
Conflicts of interest disclosure: The authors declare no competing conflicts of interests.
Correspondence: Nancy A. Speck, University of Pennsylvania, 421 Curie Blvd, Biomedical Research Building II/III, Philadelphia, PA 19104; e-mail: nancyas@upenn.edu; and Kai Tan, Children's Hospital of Philadelphia, 3401 Civic Center Blvd, Colket Translational Research Building, Philadelphia, PA 19104; e-mail: tank1@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal