Abstract
Background:
Novel therapeutic approaches with fewer and less severe side effects are needed for to treat marrow failure in patients with fanconi anemia (FA), especially as the current therapies, i.e. androgens (temporary response) or bone marrow transplantation (curative), can be associated with significant morbidity and mortality.
Studies in both animals and human samples indicate that high levels of systemic reactive-oxygen species (ROS) and increased sensitivity of hematopoietic progenitors to ROS play a key role in the pathogenesis of marrow failure associated with FA. This LPS/TNF alpha-generated hematopoietic suppression in Fancc-/- mice has been shown to respond to treatment with anti-oxidants (Sejas et al. J of Immunol, 2007). We hypothesized that treatment with the ROS scavenger quercetin (3, 30, 40, 5, 7-pentahydroxyflavone) will be safe, well tolerated and will decrease ROS levels in children with FA, which in turn will prevent or ameliorate their marrow failure.
Quercetin was chosen as a representative anti-oxidant flavonoid in this study, as it is the most abundant, natural and best studied among the dietary flavonols. In most epidemiological studies, quercetin is shown to be well tolerated without major side effects (Harwood et al., Food Chem Toxicol. 2007). We report results of our Phase 1 pilot study of safety, tolerability and pharmacokinetics (PK) of Quercetin in patients with FA.
Methods: Standard phase 1 study design was utilized and all of the required regulatory approvals including an IND were obtained. Patients were treated with oral quercetin on a twice a day dosing schedule for an initial 16 weeks (4 months) of study period, followed by continuation phase (voluntary) for an additional 20 months. Patients were monitored closely for adverse effects (AEs). Quercetin PK measurements were performed around 1st dose and again at 16 weeks. Quercetin dose was adjusted as needed based on serial assessments of Quercetin levels (per PK) and change in peripheral blood ROS for each cohort of 3 patients. Impact of Quercetin on ROS reduction and preservation of stem cell reserve were used as surrogate markers for prevention of progressive marrow failure.
Results: Twelve individuals with FA (median age of 7 years; range: 3-21), were treated with oral quercetin for 16 weeks between Aug 2012 to March 2017. All patients tolerated Quercetin well without any attributable AEs. There were no grade IV AEs. Compliance was excellent. None of the patients met stopping rules for feasibility, including those who participated in the continuation phase (up to total of 2 years of Quercetin). PK parameters for Quercetin in patients with FA were similar to previous reports in non-FA individuals (Moon et al. Biopharm. Drug Dispos. 2008).
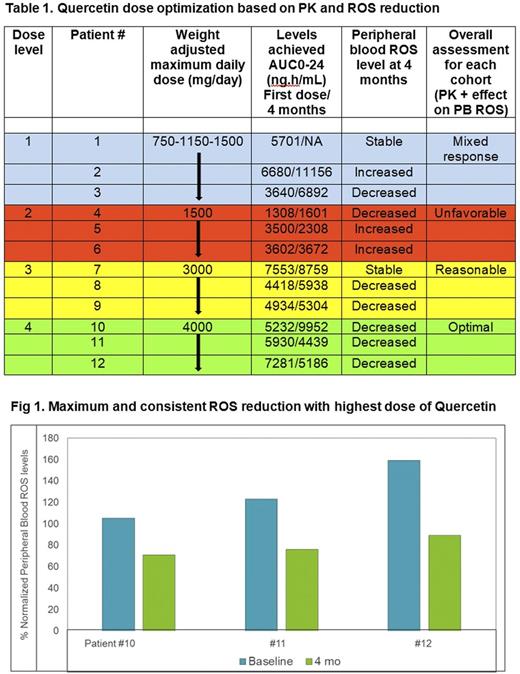
As shown in Table 1 and Fig 1, a definite trend towards peripheral blood ROS reduction was observed, especially in patients who received higher doses of Quercetin. Final weight adjusted maximum daily dose of 4000mg/day achieved the highest and most consistent ROS reduction.
ROS levels in bone marrow showed variable response. Additional correlative studies i.e. bone marrow CD34+ cell count and colony forming units (CFUs) were stable to slightly improved at 4 months compared to baseline (p=NS).
Conclusion: We conclude that long-term quercetin therapy is safe, well tolerated and can achieve biologically relevant blood levels in patients with FA. These results will form the basis of our next efficacy study, and eventually expected to advance the treatment options for patients with FA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal