Abstract
Introduction
Early relapse after frontline therapy of follicular lymphoma (FL) has been previously shown to correlate with a shorter survival. However, earlier studies were analyzed based on standard CT scan imaging. In this study, we examined the impact of early relapse on FL survival using 18FDG PET (PET) imaging.
Patients and Methods
We identified 1,120 patients (≥ 18 years) with follicular lymphoma (FL) diagnosed between 1998 and 2009 excluding those with ≤2 visits, divergent histology at initial diagnosis, and presence of concurrent malignancy. OS was defined as time of diagnosis to last follow up or death. Patients were excluded from event free survival analysis if they were never treated, or died before or during frontline treatment. Achieving event free survival at 24 mo (EFS24) was defined as being alive and event free 24 months from start of treatment. Not achieving EFS24 was defined as being alive but having an event by 24 months from start of treatment. An event was progression or change of treatment. Subsequent OS was defined as time from 24 months after start of treatment to last follow up or death and was compared between patients who achieved or did not achieve EFS24 by Kaplan-Meier method and log-rank test.
Results
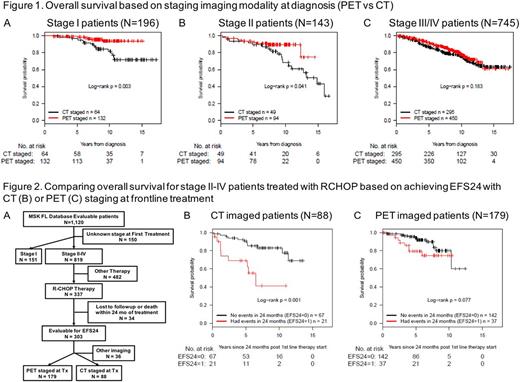
Stage at diagnosis for 1,110 patients was I, N=196; II, N=143; III-IV, N=745. Median age of diagnosis was 57 years with 51% males. With a median follow up of 8.2 years (range 0.2-17.5 years), median overall survival was not reached. At diagnosis, 676 patients were imaged with PET while 408 patients were imaged with CT. Stage I and II patients imaged with a PET at diagnosis had a significantly better OS than those imaged with a CT scan (log-rank p=0.003 and 0.041, respectively) (Figure 1A-B). In advanced stage patients (III/IV), PET versus CT imaging did not affect OS (Figure 1C). For stage I patients the 10-year OS was 82% using CT imaging and 94% using PET imaging. For stage II patients 10 year OS was 68% by CT imaging and 90% by PET imaging. We subsequently analyzed the predictive value of EFS24 using PET imaging using a subset of 303 stage II-IV FL patients treated with RCHOP as frontline therapy evaluable for EFS24 (Figure 2A). CT imaged patients demonstrated a better OS benefit for patients achieving EFS24 (Figure 2B). In contrast, the impact of EFS24 on OS was insignificant for PET imaged patients (Figure 2C). The 10 year OS for CT imaged patients was 83% (95% CI 74-93%) for those achieving EFS24, and 41% (95% CI 20-84%) for those not achieving EFS24. For patients imaged with PET prior to first treatment, 10 year OS was 80% (95% CI 69-93%) for those achieving EFS24, and 75% (95% CI 61-92%) for those not achieving EFS24.
Conclusion
Use of PET imaging is an increasingly common practice in management of lymphoma. Analyzing the overall survival of PET imaging versus CT imaged patients, PET imaging at diagnosis more accurately predicts overall survival for stage I and II patients. EFS24 as examined using CT based imaging has been proposed to inform OS in FL. In this single center subset, the predictive value of EFS24 is not observed in patients imaged with PET at the time of treatment. Therefore, EFS24 as a clinical marker for poor outcome may need to be re-examined using modern PET imaging. In conclusion, PET imaging is an important component of the management of FL for appropriate staging and assessment of prognosis.
Moskowitz: Genentech BioOncology: Consultancy; Merck: Consultancy, Research Funding; Pharmacyclics: Research Funding; Celgene: Consultancy; Seattle Genetics: Consultancy, Other: Ad Board, Research Funding. Horwitz: Kyowa-Hakka-Kirin: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; ADCT Therapeutics: Research Funding; Mundipharma: Consultancy; Celgene: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Aileron Therapeutics: Research Funding; BMS: Consultancy; HUYA: Consultancy. Zelenetz: Celgene: Consultancy; Amgen: Consultancy. Moskowitz: Seattle Genetics: Honoraria, Research Funding; ADC Therapeutics: Research Funding; Incyte: Research Funding; Takeda: Honoraria; Bristol Myers-Squibb: Consultancy, Research Funding. Gerecitano: Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Mass Medical International: Honoraria, Membership on an entity's Board of Directors or advisory committees; Orexo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Royal Bank of Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Arcus Medica: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aratana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Samus Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hamlin: Seattle Geneitcs: Other: research support; Novartis: Other: research support; Incyte: Other: research support; Gilead: Consultancy, Honoraria; Portola: Consultancy, Honoraria, Other: research support; Celgene: Consultancy, Honoraria. Kumar: Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding. Noy: Pharmacyclics LLC, an AbbVie Company: Honoraria, Other: Travel, Accommodation, Expenses, Research Funding, Speakers Bureau. Straus: Received consulting fee from Seattle Genetics for involvement in the research: Consultancy. Drullinsky: Seattle Genetics: Honoraria, Other: Ad Board. Younes: Sanofi: Honoraria; Bayer: Honoraria; Janssen: Honoraria; Roche: Consultancy, Honoraria, Other: Third-party medical writing assistance, under the direction of Anas Younes, was provided by Scott Malkin of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd.; Curis: Research Funding; Celgene: Honoraria; Seattle Genetics: Honoraria; Novartis: Research Funding; Takeda Millenium: Honoraria; Johnson & Johnson: Research Funding; Incyte: Honoraria; Bristol-Myers Squibb: Honoraria; Merck: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal