Abstract
Background
Systemic AL amyloidosis (AL) is associated with an underlying plasma cell dyscrasia and characterised by deposition of abnormally folded monoclonal immunoglobulin light chains. High dose melphalan with autologous stem cell transplantation (ASCT) can lead to durable complete haematologic and organ responses. Early studies reported TRM of >20%. Strategies for careful patient selection have made ASCT safer with TRM 2-5% but exclude ~80% of patients with AL from availing this treatment modality. Modern chemotherapy can lead to high clonal and organ responses but durability of response remains unclear. We describe our experience of deferred ASCT in 22 patients treated at two large European amyloidosis centres who were considered ineligible for ASCT at presentation but achieved clonal and organ responses with induction chemotherapy.
Methods
22 patients with AL underwent deferred ASCT from September 2011 to July 2017; 8 were treated at Heidelberg University Amyloidosis Centre and 14 were treated at the UK National Amyloidosis Centre. All underwent serial assessment of organ function, biomarkers and clonal response. Organ involvement and response were defined by international amyloidosis consensus criteria. Survival was calculated by Kaplan-Meier analysis. All analyses are on intention to treat basis (ITT).
Results
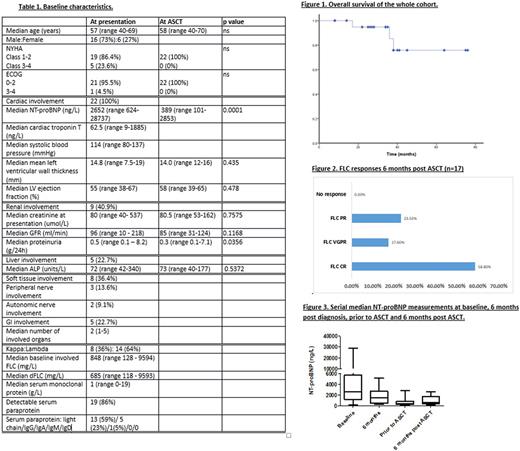
Baseline characteristics are presented in Table 1. All patients were diagnosed with AL between January 2011 - November 2016. All had cardiac involvement: Mayo Stage 2, 3A and 3B disease in 4 (18%), 16 (73%) and 2 (9%) patients respectively.
All patients received bortezomib-based induction chemotherapy, median number of cycles was 6 (range 4-9). 6 (27.2%) were treated with a bortezomib-IMID regimen, the remainder had bortezomib-dexamethasone or a bortezomib-alkylator protocol. Haematologic responses after chemotherapy on an ITT basis were: complete haematologic response (CR) - 11 (50%), very good partial response (VGPR) - 8 (36%) partial response (PR) - 3 (14%). At 6 months, 14 (64%) achieved a cardiac response, 5/9 (56%) with renal involvement achieved a renal response and 3/5 (60%) with liver involvement achieved a liver response.
Median time to ASCT from presentation was 14.5 months (range 2-71 months). Indication for ASCT was hematologic progression in 10 (45%) patients and as a consolidation procedure in the remainder. At the time of ASCT, all patients had NYHA class 1-2 symptoms (compared to 86.4% with NYHA Class 1-2 symptoms and 13.6% with NYHA Class 3-4 symptoms at presentation) and ECOG of 0-1 (compared to 95.5% with ECOG 0-2 and 4.5% with ECOG 3-4 at presentation). Median NT-proBNP was 389ng/L (101-2853ng/L)prior to ASCT, having been 2652ng/L (930-28737ng/L) at presentation (p=0.0001) and 1497ng/L (237-5167ng/L) 6 months after diagnosis (p=0.0016). Mean LV wall thickness was 14.5mm, compared to 14.2mm prior to ASCT (p=0.435). Mean LV ejection fraction was 52.5% 6 months after diagnosis, compared to 55.3% prior to ASCT (p=0.478).
Median overall survival has not been reached and 3 patients (13.6%) died (at 6, 11 and 26 months post ASCT) (Fig 1).One patient has yet to reach 3 month follow up. Number of patients in an FLC CR, VGPR, PR and no response on an evaluable basis at 3 months was as follows: 11 (52.4%), 5 (23.8%), 3 (14.3%) and 2 (9.5%). Haematologic response was assessed at 6 months post ASCT in 17 patients (5 have yet to reach 6 month follow up (data to be updated at the meeting) and 1 patient died at 6 months). The number of patients in an FLC CR, VGPR and PR at 6 months was as follows: 10/17 (58.8%), 3/17 (17.6%) and 4/17 (23.5%) respectively (Fig 2). Cardiac response at 6 months was assessed in 14 patients. Of the remainder, one had died and others are awaiting 6 month cardiac assessment. 10/14 (71.4%) were in a cardiac response 6 months post ASCT. Median NT-proBNP 6 months post ASCT was 595ng/L (189-2589 ng/L) (Fig 3).
Conclusion
This study highlights the potential role of deferred ASCT in both a consolidation or relapse setting in selected patients with cardiac AL who have achieved organ responses. No TRM was observed and ASCT was associated with good haematologic and organ responses. Younger patients (<65 years) with AL should be considered for a stem cell sparing regime to potentially avail this treatment modality if clinically significant organ responses are achieved. Longer follow up and larger studies are needed to confirm these findings and assess durability of response.
Hegenbart: Janssen: Honoraria, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Prothena: Membership on an entity's Board of Directors or advisory committees. Yong: Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Popat: Takeda: Honoraria, Other: Travel support for meetings; Amgen: Honoraria; Celgene: Honoraria, Other: Travel support for meetings; Janssen: Honoraria, Other: Travel support for meetings. Rabin: Janssen: Consultancy, Other: Travel support for meetings, Speakers Bureau; Takeda: Consultancy, Other: Travel support for meetings, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Schönland: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; prothena: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau. Wechalekar: Celgene: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal