Abstract
Data from a phase II clinical trial of venetoclax in AML recently supported its FDA breakthrough therapy designation for use in combination with hypomethylating agents in untreated AML patients ineligible for standard induction chemotherapy. Venetoclax is currently FDA approved for use in chronic lymphoid leukemia (CLL) patients with a 17(p) deletion who have been treated with at least 1 prior therapy. Del17(p) leads to loss of the TP53 gene, encoding the key tumor suppressor p53. Loss of p53 results in BCL-2 overexpression, providing a target for venetoclax's mechanism of action. Approval of this BCL-2 inhibitor in CLL raises the question of mechanism of action in AML and patient selection for treatment. Unfortunately, no biomarkers or methods exist to predict venetoclax response in AML, making treatment selection challenging.
Aim: To identify a novel genomic signature rule predicting AML response to venetoclax therapy and validate the rule using ex vivo drug sensitivity testing.
Methods: The Beat AML project is a repository of >800 bone marrow biopsy specimens and clinical data from consenting AML patients. Samples from this repository were analyzed by conventional cytogenetics, whole-exome sequencing, RNA-seq, and an ex vivo drug sensitivity assay. Refractory AML patients with sufficient data available for modeling (n=74) were randomly selected from the database, and every available genomic abnormality was entered into a computational biology program (Cellworks Group) that uses PubMed and other online resources to generate patient-specific signaling network maps of activated and inactivated protein pathways. Digital drug simulations with venetoclax were conducted by quantitatively measuring drug effect on an AML disease inhibition score, a composite of cell proliferation, viability, and apoptosis. Computational predictions of drug response were compared to venetoclax IC50 values from the Beat AML ex vivo drug sensitivity assays. For clinical relevance, 2 refractory AML patients were treated with venetoclax, and computational predictions were generated from their AML genomic abnormalities.
Results: Of the patient samples predicted by computer simulation to show sensitivity to venetoclax (n=35, 47%), 28 had the lowest IC50 values (<0.0946) of the cohort. Of the patients predicted to show resistance to venetoclax (n=39, 53%), 36 had the highest IC50 values (>1.0). Ex vivo venetoclax responses were correctly matched to their computer simulation prediction in 64 of 74 cases (86.5%), and incorrectly matched in 10 cases (13.5%). The computational method had a PPV of 80%, NPV of 92%, sensitivity of 90%, and specificity of 84%.
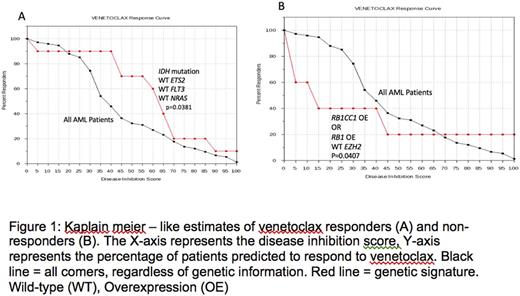
For all 74 patients in this cohort, presence of IDH mutations with absence of ETS2 overexpression and NRAS and/or FLT3 mutations improved the probability of the patient obtaining a responsive disease inhibition score, as compared to all patients in the cohort regardless of genotype (P= 0.0381) (Figure 1A). Ten patients in the cohort had a mutation in IDH, along with wild-type ETS2, NRAS, and FLT3, matching our predicted sensitivity genomic signature. We also found that mutations of PI3K or its upstream regulator, IGF1, resulted in activation of the PI3K/AKT1/mTOR pathway, indirectly increasing BCL-2 expression and suggesting sensitivity to venetoclax. Alternatively, patients with RB1 and/or RB1CC1 amplification and absence of EZH2 knockdown had a reduced probability of obtaining a responsive disease inhibition score, as compared to all patients in the cohort regardless of genotype (P= 0.0407) (Figure 1B). These rules were observed true in 2 clinical cases of AML patients treated with venetoclax, and the computational method accurately predicted clinical outcomes in both cases.
Conclusions: Computational modeling and digital drug simulation identified genomic signatures correlating with AML sensitivity or resistance to venetoclax treatment. The predictions were confirmed with an ex vivo drug sensitivity assay and clinical application in 2 AML patients. Our results will inform eligibility criteria for upcoming precision enrollment clinical trials of venetoclax in patients with AML. This technology will also be used to predict AML patient responses to a variety of pipeline and approved agents, and justifies the need for genomic sequencing in all AML patients.
Norkin: Celgene: Honoraria, Research Funding. Kumar: Cellworks: Employment. Singh: Cellworks Research India Pvt. Ltd: Employment. Kumar: Cellworks Research India: Employment. Vasista: Cellworks Research India: Employment. Abbasi: Cellworks Group Inc.: Employment. Vali: Cellworks Group Inc.: Employment. Druker: Novartis: Research Funding; ARIAD: Research Funding; Millipore: Patents & Royalties: Royalties from Dana-Farber Cancer Institute, which has an exclusive commercial license with Millipore for monoclonal antiphosphotyrosine antibody 4G10, which I developed while employed at DFCI.; Bristol-Myers Squibb: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Henry Stewart Talks: Patents & Royalties; CTI Biopharma: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Beta Cat: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; The Leukemia & Lymphoma Society: Other: Joint Steering Committee of AML Master Protocol, Research Funding; Baxalta US Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Third Coast Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Roche TCRC: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Patents & Royalties: #843 Mutated ABL Kinase Domains (licensed to various companies); #0996 Detection of Gleevec Resistant Mutations (licensed to various companies, including MolecularMD); #0606 Treatment of Gastrointestinal Stromal Tumors (exclusively licensed to Novartis); GRAIL: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; McGraw Hill: Patents & Royalties; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; MED-C: Membership on an entity's Board of Directors or advisory committees; Cylene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Monojul: Consultancy. Tyner: Incyte Corporation: Research Funding; Syros: Research Funding; Agios Pharmaceuticals: Research Funding; Seattle Genetics: Research Funding; Array Biopharma: Research Funding; Leap Oncology: Consultancy; AstraZeneca: Research Funding; Janssen Pharmaceutica: Research Funding; Takeda Pharmaceutical Company: Research Funding; Aptose Biosciences: Research Funding; Genentech: Research Funding; Constellation Pharmaceuticals: Research Funding; Gilead: Research Funding. Pollyea: Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis: Membership on an entity's Board of Directors or advisory committees; Agios, Pfizer: Research Funding. Cogle: Celgene: Other: Membership on Steering Committee for Connect MDS/AML Registry.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal