Abstract
Background
CXCL12 is a chemokine that is essential for homing of myeloid cells in the bone marrow (BM). It has been postulated that a CXCL12 receptor present in myeloid cells, CXCR4, signals in part through HRAS, a protein that is uniquely dependent on farnesylation for activity. Tipifarnib is a potent and selective inhibitor of the enzyme farnesyltransferase (FT) that had been shown to provide clinical benefit in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS); however, no molecular target that could explain the activity of tipifarnib in AML and MDS has been identified. Based on initial observations in T cell lymphoma (Witzig EHA 2017), we investigated a role for CXCL12 and myeloid cell homing in BM as biomarkers of the activity of tipifarnib in AML and MDS studies.
Methods
CTEP20 was a phase 2, multicenter, open-label study that investigated the efficacy and safety of tipifarnib in 158 older adults with previously untreated, poor-risk AML. AML301 was a phase 3 (N=457), multicenter, open-label study that evaluated the efficacy and safety of tipifarnib compared with best supportive care (BSC), including hydroxyurea, as first-line therapy in elderly patients with newly diagnosed, de novo, or secondary AML. INT28 was a multicenter phase 2 study that evaluated tipifarnib in 82 pts with poor-risk MDS. Global gene expression data generated using the Affymetrix U133A gene chip from 34 pretreatment BM samples collected from CTEP20 pts (GSE8970) was analyzed with respect to pretreatment hematological parameters and study outcomes. The Kaplan-Meier method was employed to estimate survival and progression free survival (PFS) and the analysis of prognostic variables. Clinical trial information: NCT00027872, NCT00093990, NCT00050154.
Results
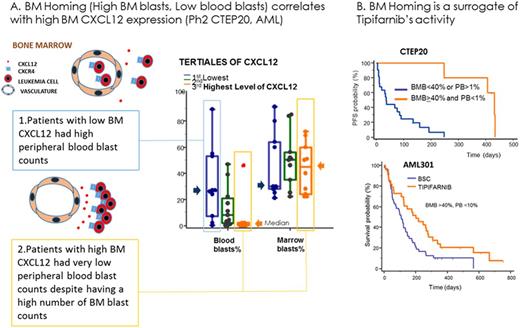
Nine complete responses (CR) and 13 best responses of progression of disease (PD) were observed in 34 pts with available BM gene expression data in study CTEP20. Pts in the 3rd (upper) tertile of CXCL12 expression experienced 6 CRs and 2 PDs whereas 1 CR and 8 PDs were observed in the 1st (lower) tertile (p=0.015). BM homing of AML blasts correlated with CXCL12 expression: Median percentage of BM blasts (BMB) and peripheral blasts (PB) were, respectively, 45% and 0% in the upper CXCL12 tertile, and 30% and 26.5% in the lower tertile (p<0.001) (Figure, A). Pts with high tumor burden in BM (BMB blasts>40%) and low circulating blasts (PB<1%) (N=5) had high CXCL12 expression in BM (3423 vs 742 counts, P<0.001, N=27) and experienced prolonged clinical benefit, with 5 CRs and a median PFS of 431 days (431 vs 35 days, HR=0.23, P<0.001, N=27) (Figure, B). The effect of BM homing was then investigated in the phase 3 AML301 trial of tipifarnib vs BSC. While the overall study (N=457) failed to identify an increased efficacy of tipifarnib compared with BSC (median survival of 109 vs 107 days, hazards ratio, HR=1.02, ns), median survival approximately doubled in the tipifarnib am compared with BSC in the subset of patients with high BMB (>40%) and low PB (<10%) while no effect was observed in the control arm (201 vs 109 days, HR=0.58, p=0.02, N=82) (Figure, B). Since CXCL12 is also a potent chemotactic factor for normal neutrophils and syndromes of hyperactivation of the CXCL12/CXCR4 pathway present with neutropenia, we investigated whether neutropenia could be a surrogate of tipifarnib activity. In AML301 pts with WBC counts>LLN (no myelosuppression), increasing neutropenia correlated with increasing clinical benefit from tipifarnib that was due in part to a poorer prognosis of BSC in neutropenic pts (113 vs 80 days median OS, HR=0.81, p=0.036, N=109, for pts with baseline neutrophils<1 giga/L). Finally, we tested the effect of neutropenia on the activity of tipifarnib in INT28. Sixty seven of the 82 MDS pts were transfusion dependent (TD) at study entry. Overall conversion to transfusion independence (TI) and response rate (ORR: CR, CRp, PR) were respectively 16% and 7.5% in the TD pts. Pts with >1 giga/L neutrophils at study entry experienced 5% TI and no responses (N=37) while 30% TI, 2 CRs, 2 CRp and 1 PR were observed in the subset of neutropenic pts.
Conclusion
CXCL12 expression and BM homing constitute potential biomarkers of the activity of tipifarnib in AML and MDS supporting the hypothesis that the CXCL12 pathway is a potential target of FT inhibitors.
Gualberto: Kura Oncology: Employment, Equity Ownership, Other: Chief Medical Officer, Patents & Royalties. Scholz: Kura Oncology: Employment, Equity Ownership, Patents & Royalties. Janes: Wellspring Biosciences: Employment. Kessler: Kura Oncology: Employment. Raza: Kura Oncology: Research Funding; Syros Pharmaceuticals: Research Funding; Novartis: Speakers Bureau; Celgene Inc.: Research Funding; Genoptix: Speakers Bureau; Onconova Therapeutics: Research Funding, Speakers Bureau; Janssen R&D: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal