Abstract
T(16;21)(p11;q22) is a rare but recurrent translocation in acute leukemias and in some types of solid tumors. The translocation leads to the formation of a FUS-ERG chimeric gene with the expression of multiple types of FUS-ERG chimeric transcripts. The prognosis of AML with t(16;21)(p11;q22) is poor in children and even worse in adults. T(16;21)(p11;q22) is also shown to be accompanied by mutations, the most frequently mutated gene was RUNX1 . We aimed to examine the mutational landscape of pediatric AML with t(16;21)(p11;q22) using an NGS-based approach.
The mutational status was analyzed with the TruSight Myeloid Panel (Illumina, San Diego, CA, USA) for the primary, remissionand relapse samples. All mutations were validated by Sanger sequencing.
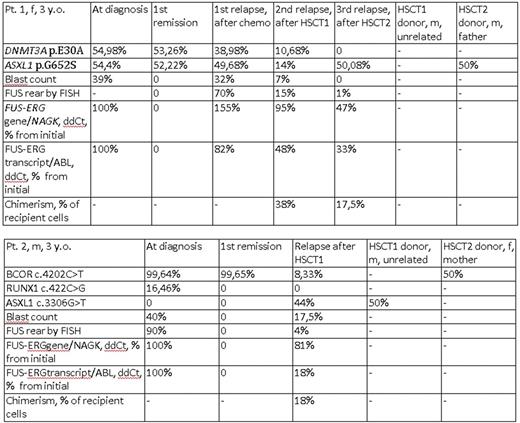
Two mutations (DNMT3A p.E30A and ASXL1 p.G652S) were found in pt. 1 initially and in the first relapse and in the second relapse. The frequency of the alternative variant correlated to the blast count and number of cells with the FUS rearrangement measured by FISH. These mutations also persisted in the first remission, when no blast cells were present and there was not a detectable level of the FUS-ERG chimeric gene and transcript. Interestingly, in the third relapse, the DNMT3A p.E30A mutation disappeared and ASXL1 p.G652S was still present. The same mutation was observed in transplant sample of patient's father who was a donor for the second HSCT.
BCOR p.P1401L and RUNX1 p.S141W mutations were present in pt. 2 initially. BCOR p.P1401L was also found in the first remission. The patient was transplanted in the first remission from HLA10/10-matched unrelated donor, but after 17 months, a relapse occurred. Here, we observed a BCOR p.P1401L mutation, no RUNX1 p.S141W mutation and a new mutation ASXL1 p.E1102D. The ASXL1 p.E1102D mutation had an alternative allele frequency that was much higher than the current blast count (44% vs 12%). This mutation was not present initially and also it disappeared after HSCT2 and its donor origin was suspected. Direct sequencing of the donor material (male, 28 y. o.) revealed ASXL1 p.E1102D. And BCOR p.P1401L mutation was also found in transplant sample of patient's mother who was a donor for the second HSCT.
We suggest that epigenetic regulator's somatic mutation DNMT3A p.E30A in pt. 1 could be the first event preceding the full transformation by t(16;21)(p11;q22)/ FUS-ERG as it persist during remission (without a chimeric gene or transcript detectable).
Findings of epigenetic regulators' mutations (ASXL1 p.G652S in pt.1 and BCOR p.P1401L in pt. 2) that are probably inherited, potentially pathogenic by computational analysis and estimated to be preleukemic in adults raise the questions if they have some influence on the emergence of primary disease and if their persistence in hematopoietic donors cells also contribute to the transplant function. It also remains to be confirmed whether unrelated unrelated donor-derived mutations (ASXL1 p.E1102D in pt. 2) actually contribute to the post-transplant relapse of the original disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal