Abstract
Introduction
VMP is a standard of care (SOC) for transplant ineligible NDMM. Daratumumab (D), a human IgGκ anti-CD38 monoclonal antibody with a direct on-tumor and multifaceted immunomodulatory mechanism of action significantly improves PFS and depth of response in combination with SOC in relapsed MM. Treatment-naïve pts may benefit greatly with the addition of D to SOC regimens. Here we report the results from the ALCYONE study, where D is added to VMP in transplant ineligible NDMM.
Methods
Pts ≥65 years or otherwise ineligible for high-dose chemotherapy with autologous stem cell transplantation were randomized 1:1 to VMP ± D and stratified by International Staging System (ISS [I, II, III]), region (Europe vs other) and age (<75 vs ≥75 years). All pts received up to a maximum of nine 6-week cycles of VMP. V: 1.3 mg/m2 SC on Days 1, 4, 8, 11, 22, 25, 29, 32 (Cycle 1) and Days 1, 8, 22, and 29 (Cycles 2-9); M: 9 mg/m2 PO and P: 60 mg/m2 PO on Days 1-4 (Cycles 1-9). In the D-VMP arm, D was given at 16 mg/kg IV QW for Cycle 1, Q3W for Cycles 2-9, and Q4W for Cycles 10+ (post VMP-treatment phase) until disease progression. The primary endpoint was PFS. Key secondary endpoints included overall response rate (ORR), rate of very good partial response (VGPR) or better, rate of complete response (CR) or better, minimal residual disease (MRD)-negativity rate (10-5 threshold, Adaptive clonoSEQ® Assay), overall survival (OS), and safety.
Results
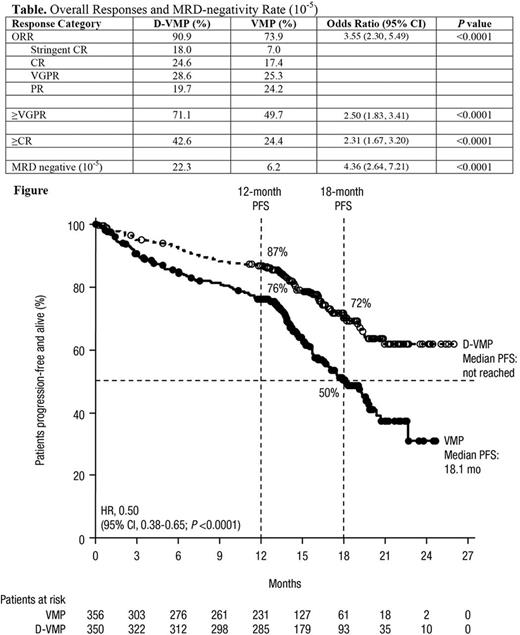
Of 706 pts randomized (350 D-VMP; 356 VMP), median (range) age was 71 (40-93) years; 29.9% were ≥75 years; 46.3% were male. 74.9% of pts had ECOG scores ≥1, and 19.3%, 42.4%, and 38.4% were ISS stage I, II, and III, respectively. Of 616 pts evaluable for FISH/karyotyping cytogenetic analysis, 84.1% and 15.9% were standard and high risk (positive for del17p, t[14;16], t[4;14]), respectively. At the timepoint of the prespecified analysis after 231 PFS events on 12 June 2017, pts had received a median (range) of 12 (1-24) vs 9 (1-9) treatment cycles for D-VMP vs VMP, respectively. 80% of pts in the D-VMP arm completed 9 treatment cycles of VMP vs 62% of pts in the VMP arm. Median (range) cumulative bortezomib doses were 46.9 (1.3-55.3) mg/m2 vs 42.2 (2.6-55.0) mg/m2 for D-VMP vs VMP, respectively.
At a median follow-up of 16.5 months, the hazard ratio for PFS (D-VMP vs VMP) was 0.50 (95% confidence interval, 0.38-0.65, P <0.0001), representing a 50% reduction in the risk of progression or death in pts treated with D-VMP (Figure). Median PFS was not reached vs 18.1 months for D-VMP vs VMP. The PFS treatment benefit of D-VMP vs VMP was consistent across all pre-specified subgroups, including age ≥75 years, ISS stage III, and high-risk cytogenetics. ORR (90.9% vs 73.9%), ≥VGPR (71.1% vs 49.7%), ≥CR (42.6% vs 24.4%) and MRD-negativity rate (22.3% vs 6.2%) were significantly higher for D-VMP vs VMP (all P < 0.0001; Table). OS data were immature after 93 deaths (45 vs 48 deaths for D-VMP vs VMP).
The most common (≥20%) all-grade treatment emergent adverse events (TEAE; D-VMP/VMP) were neutropenia (49.7%/52.5%), thrombocytopenia (48.8%/53.7%), anemia (28.0%/37.6%), peripheral sensory neuropathy (28.3%/34.2%), upper respiratory tract infection (26.3%/13.8%), diarrhea (23.7%/24.6%), pyrexia (23.1%/20.9%), and nausea (20.8%/21.5%). Most common (≥10%) grade 3/4 TEAEs (D-VMP/VMP) were neutropenia (39.9%/38.7%), thrombocytopenia (34.4%/37.6%), anemia (15.9%/19.8%), and pneumonia (11.3%/4.0%). Only 1 pt in each arm discontinued treatment due to pneumonia. The rates of grade 3/4 infections were 23.1% vs 14.7% and treatment discontinuations due to infections were 0.9% vs 1.4% for D-VMP vs VMP. D-associated infusion-related reactions (27.7%) mostly were grade 1/2 (grade 3/4, 4.3%/0.6%) and most (92.7%) occurred during the first infusion. Tumor lysis syndrome occurred in <1% of pts in each arm. Second primary malignancy occurred in 2.3% vs 2.5% pts in D-VMP vs VMP.
Conclusion
The combination of D with VMP in transplant ineligible NDMM pts doubled the PFS (HR 0.50), which was driven by more pts achieving deep responses, including significantly higher ≥CR rate and tripling of the MRD-negativity rate. No new safety signals were observed when combining D with VMP. Three phase 3 studies have now demonstrated a consistent doubling of PFS and more than threefold increase in MRD-negativity rate when combining D with SOC regimens. These results support the use of a D-based combination, D-VMP, in transplant ineligible NDMM.
Mateos:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Novartis: Consultancy, Honoraria; Genesis Pharma: Research Funding; Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology: Consultancy, Honoraria, Other: Advisory Committee: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology. Cavo:Celgene:: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria. Suzuki:Bristol Myers Squibb: Honoraria; Novarltis: Honoraria; Celgene: Honoraria; Ono Pharmaceuticals: Honoraria; Fujimoto: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Sanofi: Honoraria. Jakubowiak:Amgen Inc., BMS, Celgene, Janssen, Karypharm, Millennium-Takeda, Sanofi, SkylineDX: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; University of Chicago: Employment. Knop:Bristol-Myers Squibb Germany: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Germany: Honoraria, Membership on an entity's Board of Directors or advisory committees; AMGEN Germany: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Janssen Germany: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Germany: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Germany: Honoraria, Membership on an entity's Board of Directors or advisory committees. Doyen:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Lucio:Janssen: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Takeda: Consultancy; Roche: Consultancy. Cook:Amgen: Honoraria, Other: Travel support; Takeda: Honoraria; Myeloma UK: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria; Myeloma UK: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria; Janssen: Honoraria, Other: Travel support, Research Funding; Celgene: Honoraria, Other: Travel support, Research Funding. Garg:Janssen: Other: travel support, Research Funding, Speakers Bureau; Takeda: Other: travel support; Novartis: Other: travel support, Research Funding. Chiu:Janssen: Employment. Wang:Janssen: Employment. Carson:Janssen: Employment. Crist:Janssen: Employment. Deraedt:Janssen: Employment. Nguyen:Janssen: Employment. Qi:Janssen: Employment; Johnson & Johnson, LLC: Equity Ownership. San-Miguel:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cilag: Consultancy; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal