Abstract
CD28 and CTLA-4 are members of a family of immunoglobulin-related receptors that are responsible for various aspects of T-cell immune regulation. The family includes CD28, CTLA-4, and ICOS as well as other proteins, including PD-1, BTLA, and TIGIT. These receptors have both stimulatory (CD28, ICOS) and inhibitory roles (CTLA-4, PD-1, BTLA, and TIGIT) in T-cell function. Increasingly, these pathways are targeted as part of immune modulatory strategies to treat cancers, referred to generically as immune checkpoint blockade, and conversely to treat autoimmunity and CTLA-4 deficiency. Here, we focus on the biology of the CD28/CTLA-4 pathway as a framework for understanding the impacts of therapeutic manipulation of this pathway.
Molecular and cell biology of the CTLA-4 pathway
CTLA-4 and CD28 share 2 ligands, CD80 and CD86
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) (CD152) and CD28 are homologous receptors expressed by both CD4+ and CD8+ T cells, which mediate opposing functions in T-cell activation. Both receptors share a pair of ligands expressed on the surface of antigen-presenting cells (APCs). CD28 interacts with the CD80 dimer with relatively high affinity and the CD86 monomer with lower affinity, mediating T-cell costimulation in conjunction with T-cell receptor (TCR) signals. In contrast, interactions of the ligands with CTLA-4 serve to inhibit T-cell responses, although the precise mechanisms are not fully understood. CTLA-4 interacts with both ligands with higher affinity and avidity than CD281-3 with CTLA-4-CD80 forming the highest avidity interaction and CD28-CD86 forming the weakest interaction (Figure 1A). Among several possibilities, this raises the concept that CTLA-4 can compete with CD28 for ligand binding and thereby act as an antagonist of CD28-mediated costimulation.4,5 These interactions are thought to take place at the immune synapse between T cells and APCs where CTLA-4 has been shown to recruit CD80, thereby limiting its interactions with CD28.6,7
Schematic of CTLA-4 cell biology. (A) CTLA-4 and CD28 receptors share 2 ligands, CD80 and CD86. CD80 is a dimeric high-affinity ligand and CD86 is a monomeric lower-affinity ligand for both receptors. CTLA-4 has a higher affinity and avidity for CD80 than CD86. The relative affinities go from high to low and from left to right. (B) CTLA-4 expressed in T cells is highly endocytic. CTLA-4 is constitutively expressed in Treg or induced following T-cell activation via CD28 and TCR signaling. In the absence of the ligand, CTLA-4 is mainly found in intracellular compartments following clathrin-mediated endocytosis mediated through CTLA-4 interaction with the AP2 molecule. The AP2 (μ2 subunit) binds to the tyrosine-based (YVKM) motif of the cytoplasmic domain of CTLA-4 and mediates rapid internalization. LRBA and AP1 proteins have also been found to bind to the YVKM motif on CTLA-4, which appear to impose different fates on CTLA-4. LRBA may mediate recycling of CTLA-4 to the plasma membrane, whereas AP1 may mediate CTLA-4 trafficking to lysosomal compartments, resulting in subsequent degradation.
Schematic of CTLA-4 cell biology. (A) CTLA-4 and CD28 receptors share 2 ligands, CD80 and CD86. CD80 is a dimeric high-affinity ligand and CD86 is a monomeric lower-affinity ligand for both receptors. CTLA-4 has a higher affinity and avidity for CD80 than CD86. The relative affinities go from high to low and from left to right. (B) CTLA-4 expressed in T cells is highly endocytic. CTLA-4 is constitutively expressed in Treg or induced following T-cell activation via CD28 and TCR signaling. In the absence of the ligand, CTLA-4 is mainly found in intracellular compartments following clathrin-mediated endocytosis mediated through CTLA-4 interaction with the AP2 molecule. The AP2 (μ2 subunit) binds to the tyrosine-based (YVKM) motif of the cytoplasmic domain of CTLA-4 and mediates rapid internalization. LRBA and AP1 proteins have also been found to bind to the YVKM motif on CTLA-4, which appear to impose different fates on CTLA-4. LRBA may mediate recycling of CTLA-4 to the plasma membrane, whereas AP1 may mediate CTLA-4 trafficking to lysosomal compartments, resulting in subsequent degradation.
Although the biophysical characteristics of CD80 and CD86 are well defined, their functional differences are less clear and the ligands are often referred to together as B7 molecules or CD80/CD86. Both ligands are found on APCs, such as dendritic cells (DCs) and B cells, although they have different expression patterns, inducibility, and kinetics.8-10 Knockout mice show impaired immune responses consistent with reduced CD28 costimulation, with CD86 deficiency arguably demonstrating a more severe phenotype.9 There is considerable variation in ligand expression, making generalization difficult; CD86 is often expressed constitutively on DCs and is induced to high levels following inflammatory stimuli. In contrast, CD80 is thought to be upregulated later by DCs. Human peripheral blood monocytes express CD86 and not CD80, whereas some human T cells can express both CD80 and CD86 under different conditions of activation.11,12 Thus, at present, differences between the 2 ligands revolve mostly around different expression patterns, whereas clear functional distinctions have yet to emerge.
Cell biology of CD28
Perhaps the most surprising feature of CD28 and CTLA-4 is that although they share the same ligands, they have opposing functions. CD28 is the archetypal costimulatory molecule involved in the activation of T cells upon binding to either CD80 or CD86. Costimulatory signals from the CD28 cytoplasmic tail result from interactions with a number of signaling molecules, including the p85 subunit of PI3 kinase, the Src family kinase Lck, interleukin-2 (IL-2) inducible family kinase Itk, protein kinase C θ, and adaptor proteins such as Grb2 and GADS.13-16 These signals result in the activation of transcription factors such as NF-κB and activator protein-1 (AP-1), which are important in functional outcomes, including IL-2 production and T-cell survival. The ability of CD28 to activate NF-κB and AP-1 pathways aligns well with the concept that AP-1 is important in avoiding unresponsive exhausted states,17 which can occur in the absence of CD28 costimulation. Indeed, there is considerable overlap between the exhausted gene signature seen in CD8 T cells, resulting from an inability to recruit AP-1, and with that seen in CD4 T-cell anergy.17
In addition to the activation of signaling pathways such as PI3K, AP-1, NF-κB, and others, CD28 is increasingly thought to be important in remodeling of the actin cytoskeleton. CD28 activates actin-regulators, such as Vav-1, cofilin-1, and RLTPR,18 and actin remodeling initiated by costimulation has been shown to be required for full TCR signaling.19 The close link between CD28 and the actin cytoskeleton has been further highlighted in a recent study characterizing CD28 interaction with capZIP, a regulator of the actin cytoskeleton. This demonstrated capZIP requirement for CD28 costimulation-dependent IL-2 production.20 Effects on gene regulation are also triggered via the CD28-inducible transcription factor DEC1, required for efficient regulation of CD4+ T-cell activation pathways21 and the expression of histone acetyl transferase Ezh2, which is responsible for maintaining the regulatory T-cell (Treg) program.22 Thus, CD28 signaling pathways are important for both effector T-cell activation and Treg function.
Cell biology of CTLA-4
Although CD28 is constitutively expressed on the plasma membrane and costimulates T cells, CTLA-4 is predominantly found in intracellular vesicles in FoxP3+ Treg cells or activated conventional T cells. This localization is due to the constitutive endocytosis of CTLA-4 from the plasma membrane and results in ∼90% of CTLA-4 being intracellular.23,24 The endocytosis of CTLA-4 is extremely rapid, with >80% of surface CTLA-4 being internalized within 5 minutes.25 Once internalized, CTLA-4 molecules appear to be either recycled to the plasma membrane or degraded in lysosomal compartments (Figure 1B); however, the details of this process and its functional significance are not yet fully understood.
The postendocytic fate and control of CTLA-4 trafficking is still poorly characterized, although experiments show that CTLA-4 lacking its 36-amino-acid cytoplasmic tail is predominantly located at the cell surface.26 A number of studies have therefore focused on identifying partners that interact with the cytoplasmic tail of CTLA-4 and on defining the role of its cytoplasmic domain. CTLA-4 endocytosis is dependent on clathrin because of its interaction with the μ2 subunit of the clathrin adaptor protein complex AP224,27 and is also dependent on dynamin. AP2 targets the cytoplasmic tyrosine containing the YVKM motif of CTLA-4 and can be disengaged when this motif is tyrosine-phosphorylated upon T-cell activation.24,28 However, the immunological settings where the CTLA-4-AP-2 interaction is disrupted are unclear because activated T cells and Treg continue to endocytose CTLA-4.25 The cytoplasmic domain sequence and trafficking behavior of CTLA-4 are highly conserved in mammals, whereas some animals such as fish lack this endocytic motif,5,29,30 resulting in predominantly surface expression. This suggests that endocytosis may be a later adaptation of CTLA-4. Overall, CD28 and CTLA-4 differ profoundly in subcellular location despite binding to the same ligands and, given the conservation of these features, it seems likely they are pivotal to the functioning of the system.
The significance of CTLA-4 trafficking was recently highlighted in patients with lipopolysaccharide-responsive and beige-like anchor protein (LRBA) deficiency.31 This Beige and Chediak-Higashi (BEACH) domain containing protein32 appears to regulate CTLA-4 turnover, and it colocalizes predominantly with recycling (Rab11+) endosomes.31 Loss of LRBA leads to increased CTLA-4 degradation with associated autoimmunity, indicating that LRBA may inhibit CTLA-4 trafficking to lysosomes and promote its recycling. This may be achieved through LRBA binding to the YVKM sequence of CTLA-4 (Figure 1B). Additional control of CTLA-4 trafficking and cellular distribution may also be mediated via interaction with the TRIM/LAX/Rab8 complex, which is involved in the post-Golgi transport of CTLA-4 to the cell surface.33 More recently, CTLA-4 interactions involving the PKC-η and PIX-PAK pathway have also been reported. These interactions appear to modulate Treg-APC interactions: PKC-η−deficient Treg are associated with impaired depletion of CD86 from APCs by Treg and increased Treg motility.34
Transendocytosis: a cell-extrinsic molecular mechanism of CTLA-4 function
A number of different models have been proposed to explain the mechanism of CTLA-4 function. The simplest of these involves competition between CD28 and CTLA-4 for ligand binding,4 which has been proposed alongside other cell-intrinsic inhibitory signaling models.26,35,36 However, although CD4 T cells from CTLA-4–deficient mice have a hyperactivated, disease-causing phenotype, the presence CTLA-4–expressing cells in chimeras can prevent disease and normalize the phenotype of CTLA-4–deficient cells via a dominant, cell-extrinsic mechanism.37
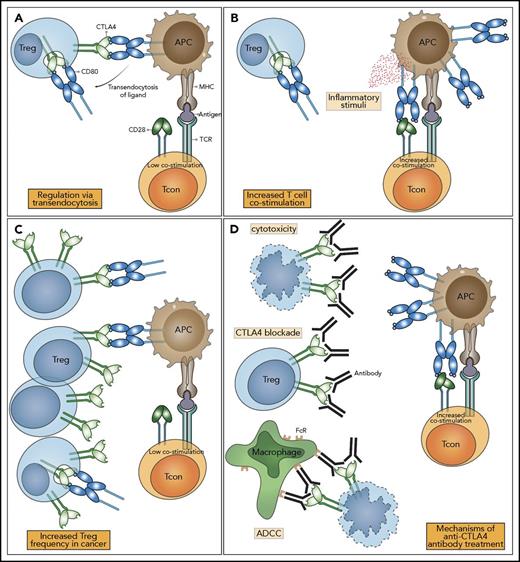
One molecular mechanism that is consistent with a dominant cell-extrinsic role for CTLA-4 is the physical capture of CD80 and CD86, and their subsequent removal from APCs: a process known as transendocytosis38 (Figure 2A). Transendocytosis requires T-cell recognition of peptide and represents an antigen-specific mechanism for controlling CD80 and CD86 expression levels on APC. Although the molecular mechanisms underlying transendocytosis itself are still largely unknown, the CTLA-4 pathway is not the only receptor-ligand pair that utilizes such a pathway. Other examples include the Notch-Δ pathway,39,40 where binding of Δ (ligand) allows subsequent removal of Notch (receptor) from the adjacent cell. Similar examples operating in the absence of ectodomain cleavage include the gap junction protein connexin-43,41 adhesion junctions via VE-cadherin,42 receptor tyrosine-kinases Eph-ephrin interaction,43 and CD47-SHPS-1.44 Although these examples are present in varied biological systems, their overall similarity suggests that a conserved molecular machinery capable of removing membrane-bound molecules from neighboring cells exists.
CTLA-4 function and the impact of immune checkpoint blockade. (A) In health, Tregs express CTLA-4, which binds CD80 and CD86 expressed on APCs. CTLA-4 binds to CD80 and CD86 with higher affinity and avidity than does CD28, preventing conventional T cell (Tcon) stimulation through CD80/CD86 interaction with CD28. Removal of CD80/CD86 ligands by transendocytosis results in impaired costimulation of T cells via CD28, resulting in immune regulation. (B) When the immune system is stimulated in the presence of inflammatory/innate immune stimuli, APCs upregulate the expression of CD80/CD86, overcoming their control by Treg, enabling costimulation and the proliferation of T cells. (C) In the tumor microenvironment, CD80/CD86 is controlled by Treg, and the abundance of Treg leads to the suppression of immune responses. (D) Anti–CTLA-4 antibodies bind to CTLA-4 molecules with high affinity, leading to Treg depletion or functional blockade, resulting in enhanced T-cell activation and immunological responses to cancer. The impacts of CTLA-4 blockade can be mediated by a variety of mechanisms: prevention of transendocytosis increasing CD80/CD86 levels on APCs, direct Treg cytotoxicity, and antibody-dependent cellular cytotoxicity mediated by FcR-IV expressing intratumoral macrophages. Note: Only CD80 is shown here for clarity. ADCC, antibody-dependent cellular cytotoxicity; MHC, major histocompatibility complex.
CTLA-4 function and the impact of immune checkpoint blockade. (A) In health, Tregs express CTLA-4, which binds CD80 and CD86 expressed on APCs. CTLA-4 binds to CD80 and CD86 with higher affinity and avidity than does CD28, preventing conventional T cell (Tcon) stimulation through CD80/CD86 interaction with CD28. Removal of CD80/CD86 ligands by transendocytosis results in impaired costimulation of T cells via CD28, resulting in immune regulation. (B) When the immune system is stimulated in the presence of inflammatory/innate immune stimuli, APCs upregulate the expression of CD80/CD86, overcoming their control by Treg, enabling costimulation and the proliferation of T cells. (C) In the tumor microenvironment, CD80/CD86 is controlled by Treg, and the abundance of Treg leads to the suppression of immune responses. (D) Anti–CTLA-4 antibodies bind to CTLA-4 molecules with high affinity, leading to Treg depletion or functional blockade, resulting in enhanced T-cell activation and immunological responses to cancer. The impacts of CTLA-4 blockade can be mediated by a variety of mechanisms: prevention of transendocytosis increasing CD80/CD86 levels on APCs, direct Treg cytotoxicity, and antibody-dependent cellular cytotoxicity mediated by FcR-IV expressing intratumoral macrophages. Note: Only CD80 is shown here for clarity. ADCC, antibody-dependent cellular cytotoxicity; MHC, major histocompatibility complex.
In summary, CTLA-4 is a highly endocytic molecule that binds to 2 distinct ligands, CD80 and CD86, leading to their removal from opposing cells. By utilizing transendocytosis, CTLA-4 can act as an immune regulatory mechanism by directly reducing the ability of APC to stimulate via CD28. This concept helps explain why a stimulatory and an inhibitory receptor share the same ligands and embraces the endocytic nature of CTLA-4. Taken together, this model suggests that the CD28/CTLA-4 system functions as a rheostat that can tune T-cell activation up or down.
CD28 and CTLA-4: a volume control on T cell responses?
The use of a random repertoire of antigen receptors by T cells provides effective coverage for the recognition of evolving pathogens. However, it generates a significant problem: that of preventing such receptors recognizing self-tissues. Such self-reactivity can lead to potentially fatal autoimmune conditions, including type 1 diabetes, inflammatory bowel disorders, autoimmune cytopenias, and others. Although T-cell selection in the thymus can remove some of these self-reactive specificities, it is incomplete for a variety of reasons.45-47 Therefore, significant numbers of potentially autoreactive T cells are present in the circulation of healthy individuals, at frequencies similar to other antigen specificities.48 Indeed, recent evidence suggests that tolerance to some tissue-restricted self-antigens cannot be achieved by deletion and relies on the presence of Tregs.49 Thus, although thymic selection mitigates T-cell self-reactivity, additional controls are required that involve the CD28/CTLA-4 system.
CD28 function: turning up the volume
The requirement for CD28 to costimulate T-cell activation emerged from studies by Schwartz and Jenkins.50 Here, antigen-specific T cells entered an unresponsive state, termed T-cell anergy, in response to TCR stimuli in the absence of a “second signal.” This signal was subsequently identified as coming from the CD28 receptor interacting with CD80 or CD86. Because these ligands are upregulated by a variety of inflammatory signals (Figure 2B), CD28 signaling provides information on the inflammatory context or “danger” accompanying TCR recognition. Importantly, CD28 costimulation appears to be additive with signals via the TCR enhancing commitment to, as well as more rapid, cell division.51 Moreover, the requirement for CD28 costimulation is related to strength of TCR signaling52 such that strong TCR stimulation requires less costimulation.53-55 Thus, weak TCRs (such as those that are self-reactive) may be effectively controlled by constraining the availability of CD28 costimulation. These concepts relating to control of CD28 costimulation become significant when considering how CTLA-4 might function to quantitatively limit CD28 signaling,56 thereby controlling self-reactive and tumor-reactive T cells in the absence of inflammation.
The importance of CD28 signaling in driving T-cell proliferative responses has been strikingly reinforced by the recent identification of CD28 mutations in T-cell leukemias that increase its affinity for ligands. Here, point mutations with increased ligand affinity as well as direct CTLA-4-CD28 fusions have been observed, generating high-affinity CD28 variants,57,58 which presumably are less effectively out-competed by CTLA-4. To date, a large body of work indicates that CD28 signals drive critical T-cell effector functions by increasing the magnitude of T-cell responses, stimulating metabolic changes59,60 as well as enhancing T-cell differentiation61,62 and survival.63 CD28 signals also contribute to enhanced cytokine production and influence T-cell migration64 and memory T-cell responses to infections.65,66 Given these potent activating functions, it is apparent that effective control of CD28 costimulation is essential and can be provided by CTLA-4.
CTLA-4: turning down the volume
The observation of fatal autoimmunity in CTLA-4 knockout mice67,68 resulting from the release of self-reactive T cells69 illustrated that CTLA-4 was a critical negative regulator of T-cell responses. T cells isolated from knockout mice showed high levels of activation markers, including CD25. Although the initial interpretation of this activated T-cell phenotype focused on the possibility that CTLA-4 mediated an inhibitory signal preventing T-cell activation,70 accumulating evidence now points to an important role for CTLA-4 in mediating the suppressive functions of Treg. Indeed, early experiments indicated that the major autoimmune phenotype of CTLA-4–deficient mice could be prevented by the presence of CTLA-4–expressing cells in mixed chimeras.37 This observation has been supported by a number of other experiments (see Walker and Sansom5 ), and recently, a series of conditional and inducible CTLA-4 deletion experiments have confirmed that the major phenotype is consistent with an effector function for CTLA-4 on Treg.71-73 These data demonstrate that CTLA-4 on Treg can control the activity of other cells (such as APCs or naive T cells), thereby controlling fatal autoimmunity (Figure 2A).
Although CTLA-4 on Treg is clearly important in preventing autoimmunity, its expression is also induced on activated T cells. Evidence from knockout mice demonstrates a more severe phenotype in complete CTLA-4 knockouts compared with Treg-specific knockouts, indicating an important role for CTLA-4 in conventional T cells.72 However, the function of CTLA-4 in non-Tregs can also be cell extrinsic, suggesting they might use the same mechanism as Treg,74,75 and activated Tcon can carry out transendocytosis.38 Nonetheless, cell-intrinsic functions of CTLA-4 controlling the homeostatic expansion of conventional T cells have been observed in vivo, although in vitro responses to stimulation were found to be similar between wild-type and knockout conventional T cells.73
It is also clear that CTLA-4 and CD28 directly influence Treg homeostasis. For example, CD28-deficient mice, or those where ligands are blocked, have reduced Treg numbers because of altered Treg generation and peripheral survival.76-79 Conversely, the loss of CTLA-4 from Treg promotes their expansion via increased CD28 signaling.80,81 Thus, both CD28 and CTLA-4 profoundly impact the generation, maintenance, and function of Treg. This intimate connection between CD28 and CTLA-4 function means that it is difficult to understand manipulation of 1 receptor without considering the impact on the other. Indeed, it is arguable that understanding the impact of CTLA-4 manipulation is best appreciated in terms of its ultimate effect on CD28-CD80/CD86 interactions.
Targeting the CTLA-4 pathway: lessons from the clinic
Targeting the CD28/CTLA-4 pathway using antibodies and fusion proteins is of considerable interest in the treatment of cancer and autoimmune diseases. Because CTLA-4 limits immune responses to self-tissues, augmenting this pathway represents a treatment option for autoimmunity, whereas suppressing this has the potential to stimulate anti–self-responses toward tumors. The potential for the immunological rejection of cancer by harnessing immune responses was first realized using anti–CTLA-4 antibodies and has resulted in exponential activity in this area.82
Initially, murine cancer models demonstrated that anti–CTLA-4 antibody treatment resulted in tumor regression and relative resistance to reinoculation with cancer.83 Less immunogenic cancers were, however, unresponsive to anti–CTLA-4 therapy unless granulocyte-macrophage colony-stimulating factor–producing vaccines were coadministered.84 Importantly, enhanced antitumor responses following CTLA-4 blockade were associated with autoimmunity. This was illustrated by CD8 T-cell–mediated depigmentation in melanoma models,85 immune-mediated prostatitis following vaccination against prostate cancer-specific antigens,86 and the generation of anti–double-stranded DNA antibodies.87 Based on the efficacy of CTLA-4 blockade in animal models, anti–CTLA-4 antibodies were developed for clinical use. Ipilimumab, a recombinant human immunoglobulin G1 (IgG1) monoclonal antibody, and tremelimumab, a human IgG2 monoclonal antibody (previously known as ticilimumab), have both been trialed in a range of advanced stage cancers. It is clear that ipilimumab potently blocks the interaction of CTLA-4 with its ligands by binding to the MYPPPY motif, which is critical for this interaction.88 Reports from human trials in melanoma, non–small cell lung cancer, mesothelioma, prostate, ovarian, breast, and urothelial cancer treatment have shown efficacy.89-108 Alongside the benefits in tumor control, these trials nonetheless demonstrate a broad range of immune related adverse events (irAEs) occurring in 60% to 65% of patients. irAEs most commonly affect the skin, gastrointestinal (GI) tract, and endocrine organs.109 In addition, autoimmunity has been reported in almost all other organs, including the liver, eyes, nervous system, lungs, heart, kidneys, and joints.89-108,110,111 The breadth of irAEs is therefore consistent with the biological role of CTLA-4 in the maintenance of polyclonal immune self-tolerance.
Further evidence for a role of CTLA-4 in the control of polyclonal autoreactive T cells has come from examining the circulating TCR repertoire following anti–CTLA-4 therapy. Increased diversity of the TCR repertoire occurs following treatment,112 associated with irAE frequency,113 and polyclonal T-cell expansion predates and predicts irAEs.114 These data are consistent with the idea that CTLA-4 blockade allows the expansion and activation of new T-cell clones and fits with the idea that self-reactive T cells are present in healthy individuals48,115 and are prevented from expanding by CTLA-4–mediated control of CD80/CD86.116
The fact that CTLA-4 blockade enables immune responses to cancers and healthy self-tissues indicates that significant recognition of self-antigens exists within our immune repertoire. This is supported by data from heterozygous loss-of-function mutations in the CTLA-4 gene. Individuals with these mutations present with an autosomal dominant syndrome of complex autoimmunity and immunodeficiency.81,117 This phenotype has many similarities to anti–CTLA-4 antibody treatment and includes enteropathy, autoimmune cytopenias, hemolytic anemia, thyroid disease, arthritis, psoriasis, granulomatous lung disease, and lymphocytic infiltration of nonimmune organs. Furthermore, a second CTLA-4 deficiency syndrome caused by autosomal recessive mutations in the LRBA gene is associated with an earlier onset, but phenotypically similar autoimmune syndrome,31,118,119 consistent with the role played by LRBA in the trafficking of CTLA-431 (Figure 1B). The similarities in wide-ranging autoimmunity between genetic deficiency in the CTLA-4 pathway and the impact of anti–CTLA-4 treatments are striking and reaffirm the central role for CTLA-4 in the maintenance of self-tolerance seen in animal studies.
The concept that CTLA-4 deficiency leads to a CD28-driven disease is also supported in humans by the effect of treatment with the CTLA-4–Ig fusion protein, abatacept. This drug has an established role in the treatment of autoimmune disorders, including rheumatoid arthritis,120 where it is viewed through the lens of blocking the CD28 costimulation pathway. In contrast, in CTLA-4 deficiency syndromes, abatacept is viewed as a CTLA-4 replacement therapy. Nonetheless, the functional effects are the same in that CD80 and CD86 ligand availability is diminished. The fact that CTLA-4– and LRBA–deficient patients respond to abatacept31,121 is consistent with the concept that these diseases are driven by excessive CD28 costimulation and in agreement with the fundamental biology of CTLA-4, as described above.
Individuals with a mutation in a single allele of CTLA-4 are highly susceptible to autoimmunity, raising the possibility that more subtle variations in the level of CTLA-4 in the general population may influence responses to treatment. Indeed, nonpathogenic polymorphisms in CTLA-4 that alter the expression of CTLA-4 influence an individual’s lifetime risk for the development of cancer and autoimmune disorders.122 Taken together, these findings illustrate that CTLA-4 expression has a pivotal role in balancing immune responses against self-tissues, and therefore, in regulating both autoimmune and anticancer responses.
The impact of CTLA-4 blockade on Treg biology
Because Tregs constitutively express CTLA-4 at high levels, anti–CTLA-4 therapy would be expected to have the greatest effect on these cells. Data from human trials are mixed, but an increased absolute number of circulating FoxP3+ CD4 Tregs have been observed in some studies.104,123,124 Similarly, CTLA-4 haploinsufficiency results in either preserved or increased Treg numbers, because of a lack of CTLA-4 and resultant increased CD28 signaling.81,117,125 Indeed, the role of CD28 signaling has been exploited in Treg expansion via the use of CD28-agonist antibodies. Although initial trials of this strategy were associated with significant inflammatory side effects, recent efforts have shown promise.126,127
Although Tregs express CTLA-4 at higher levels, activated conventional T cells do express CTLA-4, and there is additional evidence from cancer therapies to suggest conventional T-cell CTLA-4 may have an important role in self-tolerance. Anti–CTLA-4 treatment combined with Treg depletion was shown to be more effective at inducing both antitumor responses and autoimmunity in animal models.128 Also, using selective blockade of CTLA-4 on Treg or conventional T cells, it was demonstrated that Treg CTLA-4 blockade alone could not induce antitumor immunity, although it could augment the antitumor responses induced by CTLA-4 blockade of conventional T cells.129 These experiments suggest that CTLA-4 expressed by conventional T cells is important in control of polyclonal immune self-tolerance, and the greater number of conventional T cells may compensate for their lower levels of CTLA-4 expression compared with Treg, when considering the functional impact of CTLA-4.
Despite the fact that limiting CTLA-4 function can drive an increase in Treg numbers, there are indications that reducing tumor-infiltrating Tregs may be important in determining the response to immunotherapy (Figure 2C). A reduction in tumor infiltrating FoxP3+ CD4 Treg was observed in one study following anti–CTLA-4 treatment95 (Figure 2D), although not in another.98 However, a possible mechanism of Treg control by anti–CTLA-4 therapies is Treg depletion via antibody-dependent cellular cytotoxicity. Here, anti–CTLA-4 antibodies bound by FcR-IV expressing intratumoral macrophages results in removal of Treg130 (Figure 2D).
CTLA-4 and the microbiome
One interesting observation from clinical blockade of CTLA-4 is that the most frequent and earliest sites of irAE are at the microbe-rich barriers of the skin and gut,131 suggesting either that CTLA-4 has a particularly important role at these sites or that its biology is influenced by microbial exposure. Fluctuating levels of antibodies against commensal GI microbes were observed in patients following anti–CTLA-4 treatment,132 suggesting dysregulated mucosal immunity with CTLA-4 blockade. This interaction of the GI microbiome and CTLA-4 function has had considerable attention recently given that tumor control and effector immune responses following anti–CTLA-4 therapy are impaired in germ-free or antibiotic-exposed mice.133 Reconstitution of the mouse GI microbiome with specific Bacteroides species or with transplantation of Bacteroides-predominant fecal microbes from patients restored antitumor responses. Improved tumor responses following anti–CTLA-4 therapy are associated with particular microbial populations and also with a reduced incidence of colitis in mice133 and patients.134 Similarly, individuals with CTLA-4 deficiency also tend to experience immune targeting of microbe-rich sites, including the skin and gut; however, studies of microbiota have yet to be reported. Taken together, these findings suggest that the microbial environment in the gut modulates the impact of CTLA-4 activity in the control of both distant tumor and local self-tissue responses.
Correlations between poor antitumor responses, higher peripheral Treg, and gut homing lymphocyte numbers along with specific microbiome clusters have been described. In patients with a microbiome cluster associated with better clinical responses, greater upregulation of ICOS (which is associated with more robust clinical responses) was observed on CD4 cells following treatment.134 Treg from mice raised under germ-free conditions were found to be impaired in their capacity to suppress T-cell proliferation, and the CD25+ CD4 cells from mesenteric lymph nodes of germ-free mice less frequently express FoxP3 or CTLA-4.135 Taken together, these findings suggest that the microbiome is important in influencing effector and Treg responses and potentially the dependence of mucosal immune regulation on CTLA-4. However, if CTLA-4 plays a critical role in GI immune regulation, a benefit of CTLA-4-Ig might have been expected in inflammatory bowel diseases, but this has not been demonstrated.136
Conclusions
The CTLA-4 pathway is a critical regulator of T-cell responses to tissues. The sharing of ligands with the stimulatory receptor CD28 establishes a rheostat, capable of tuning T-cell responses. Accordingly, decreasing CTLA-4 function by antibody blockade, or in CTLA-4 genetic disorders, increases the availability of ligands for CD28, permitting the activation of potentially self-reactive T cells and altering Treg homeostasis. Furthermore, the high-level expression of CTLA-4 on Treg and the emerging cell biology of this pathway continue to offer opportunities to stimulate or inhibit T-cell responses.
Acknowledgments
This work was funded by the Biotechnology and Biological Sciences Research Council (B.R.) and by the Wellcome Trust Programme for Clinicians (N.H.).
Authorship
Contribution: B.R., N.H., and D.M.S. cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Sansom, UCL Institute of Immunity and Transplantation, Royal Free Hospital, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: d.sansom@ucl.ac.uk.
References
Author notes
B.R. and N.H. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal