TO THE EDITOR:
Despite improvements in treating childhood leukemia, relapses remain the primary cause of death.1-4 On relapse, leukemic cells are more resistant to chemotherapy. Thus, there is an urgent need to incorporate new strategies to treat childhood leukemia. Aberrant DNA methylation has been strongly associated with progression, relapse, and drug resistance.5-10 These observations support the rationale to incorporate hypomethylating agents, such as azacitidine (AZA) and decitabine, in antileukemia therapy. At lower doses, these agents act as DNA methyltransferase inhibitors (DNMTis), inducing global DNA hypomethylation, reactivating tumor suppressors, downregulating oncogenes, stimulating an innate antiviral immune response, and increasing sensitivity to cytotoxic agents.11-17
Based on these observations, the Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) consortium conduced a phase 1 study to administer 5 days of AZA (75 mg/m2 per day, subcutaneously) followed by 5 days of fludarabine (30 mg/m2 per day) and cytarabine (2 g/m2 per day) in children with relapsed/refractory leukemia. After the safety evaluation of this combination, an additional 8 patients with acute myeloid leukemia (AML) were included in an expansion cohort. The study was approved by the institutional review boards of participating institutions. Informed consent was obtained from all subjects or guardians. This study was registered at www.clinicaltrials.gov as #NCT01861002.
Fourteen patients (AML: n = 12; acute lymphoblastic leukemia [ALL]: n = 2) were enrolled, and all were evaluable for toxicity and response. The median number of previous treatment regimens was 2 (range: 1-5). Five patients had previously undergone hematopoietic stem cell transplantation (HSCT). Four patients (28.6%) were refractory to the most recent treatment. Detailed eligibility criteria, treatment, and patient characteristics are listed in the supplemental Methods and supplemental Table 1, available on the Blood Web site.
Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. They were typical of intensive chemotherapy regimens (Table 1). Leukopenia and infection were the most common adverse events (AEs) observed. None of the patients experienced dose-limiting toxicity or elevation of peripheral white blood cells during the AZA “priming” period. Ten of 14 patients received 2 courses of therapy. The median duration from the initiation of course 1 to the start of course 2 was 43 days (range: 34-48 days). The reasons for not receiving a second course were: progressive disease (n = 3) and HSCT after course 1 (n = 1).
Grade 3 or higher nonhematological AEs
| . | Course 1 (n = 14) . | Course 2 (n = 10) . | ||||||
|---|---|---|---|---|---|---|---|---|
| AEs regardless of attribution, n (%) . | AEs attributed to AZA,n (%) . | AEs regardless of attribution, n (%) . | AEs attributed to AZA,n (%) . | |||||
| Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | |
| Investigations | ||||||||
| White blood cell count decreased | 0 (0) | 13 (93) | 0 (0) | 10 (71) | 0 (0) | 8 (80) | 0 (0) | 5 (50) |
| Lymphocyte count decreased | 1 (7) | 3 (21) | 1 (7) | 2 (14) | 0 (0) | 2 (20) | 0 (0) | 1 (10) |
| Infection | ||||||||
| Febrile neutropenia | 8 (57) | 0 (0) | 5 (36) | 0 (0) | 6 (60) | 1 (10) | 4 (40) | 1 (10) |
| Sepsis | N/A | 0 (0) | N/A | 0 (0) | N/A | 1 (10) | N/A | 0 (0) |
| Infections and infestations, other | 3 (21) | 0 (0) | 2 (14) | 0 (0) | 5 (50) | 0 (0) | 2 (20) | 0 (0) |
| Gastrointestinal | ||||||||
| Anorexia | 2 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Mucositis oral | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Oral hemorrhage | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Metabolic/laboratory | ||||||||
| Hyponatremia | 2 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AST/ALT elevation | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 3 (30) | 0 (0) | 2 (20) | 0 (0) |
| Blood bilirubin increased | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypokalemia | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypophosphatemia | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| General disorder | ||||||||
| Pain | 1 (7) | N/A | 0 (0) | N/A | 1 (10) | N/A | 0 (0) | N/A |
| . | Course 1 (n = 14) . | Course 2 (n = 10) . | ||||||
|---|---|---|---|---|---|---|---|---|
| AEs regardless of attribution, n (%) . | AEs attributed to AZA,n (%) . | AEs regardless of attribution, n (%) . | AEs attributed to AZA,n (%) . | |||||
| Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | |
| Investigations | ||||||||
| White blood cell count decreased | 0 (0) | 13 (93) | 0 (0) | 10 (71) | 0 (0) | 8 (80) | 0 (0) | 5 (50) |
| Lymphocyte count decreased | 1 (7) | 3 (21) | 1 (7) | 2 (14) | 0 (0) | 2 (20) | 0 (0) | 1 (10) |
| Infection | ||||||||
| Febrile neutropenia | 8 (57) | 0 (0) | 5 (36) | 0 (0) | 6 (60) | 1 (10) | 4 (40) | 1 (10) |
| Sepsis | N/A | 0 (0) | N/A | 0 (0) | N/A | 1 (10) | N/A | 0 (0) |
| Infections and infestations, other | 3 (21) | 0 (0) | 2 (14) | 0 (0) | 5 (50) | 0 (0) | 2 (20) | 0 (0) |
| Gastrointestinal | ||||||||
| Anorexia | 2 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Mucositis oral | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Oral hemorrhage | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Metabolic/laboratory | ||||||||
| Hyponatremia | 2 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AST/ALT elevation | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 3 (30) | 0 (0) | 2 (20) | 0 (0) |
| Blood bilirubin increased | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypokalemia | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypophosphatemia | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| General disorder | ||||||||
| Pain | 1 (7) | N/A | 0 (0) | N/A | 1 (10) | N/A | 0 (0) | N/A |
ALT, alanine transaminase; AST, aspartate transaminase; N/A, not applicable.
Neither of the 2 patients with ALL responded. Of the 12 AML patients, 7 achieved complete remission (CR; n = 6) or CR with incomplete count recovery (CRi; n = 1) after course 1, including three patients who had previous HSCT (supplemental Tables 2 and 3). Three patients with partial response (PR)/stable disease (SD) received a second course, and none of them responded. For the patients who achieved CR, the median time for a post-nadir absolute neutrophil count ≥1,000/µL and platelet count ≥100 000/µL was 47 days (range: 42-65 days) and 39 days (range: 34-65 days), respectively.
This is the first study to test the DNMTi, AZA, combined with intensive chemotherapy in pediatric acute leukemia. We showed that this combination is well tolerated. Toxicities were typical for patients receiving intensive chemotherapy, and no new or unexpected toxicities were observed, suggesting the priming strategy could be safely used in this previously heavily treated population.
The CR/CR with incomplete blood count recovery (CRi) rate in AML patients was 58%. Four of 7 patients who achieved CR/CRi also attained minimal residual disease–negative status (<0.1% centrally reviewed by flow cytometry). Although recognizing that the small sample size does not allow for significant comparison with published childhood AML relapsed studies,2,4 these results are encouraging considering that the majority (8 of 12) of the AML patients had ≥2 previous salvage treatment attempts.
To evaluate the effect of AZA, blood from all patients was obtained at baseline (day 0) and within 24 hours after the last dose of AZA (day 6). A reduction of GLOBAL-LINE (G-LINE) methylation was noted in all patients after AZA treatment, suggesting genome-wide methylation changes. However, no difference in G-LINE methylation was observed between responders vs nonresponders at baseline or post–AZA treatment (supplemental Figure 2).
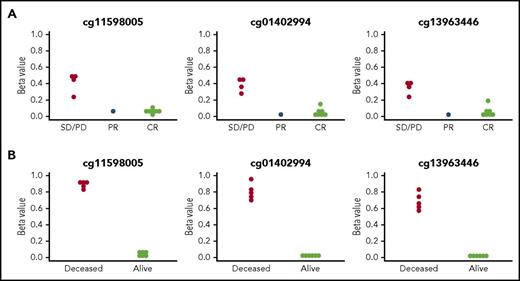
Next, we performed comprehensive DNA methylation profiling using the HM450 DNA methylation array in the bone marrow (BM) of 12 AML patients. A supervised analysis revealed a distinct methylation pattern at baseline between the patients who responded to therapy and nonresponders. We therefore used DMRcate,18 a smoothing procedure (supplemental Methods), to find significant differences in DNA methylation across contiguous regions. After adjusting for multiple comparisons, a single region intronic to known transcripts of the farnesyldiphosphate farnesyl-transferase 1 (FDFT1) gene showed significant differential methylation between patients with PR, CR/CRi, and without response (Figure 1A).
Baseline methylation status of CpG loci in the FDFT1 gene region. There are 3 CpG probes located in this region on the Infinium HumanMethylation450 array. (A) Methylation status of the FDFT1 gene region in the peripheral blood of AML patients enrolled in the TACL trial at baseline. P = .0021 across all 3 CpGs, by Fisher’s exact test. (B) Methylation status of the FDFT1 gene region in BM or peripheral blood (as available) from patients enrolled in the D-ADE arm of the DACOGEN202 trial at baseline. P = .0022. PD, progressive disease.
Baseline methylation status of CpG loci in the FDFT1 gene region. There are 3 CpG probes located in this region on the Infinium HumanMethylation450 array. (A) Methylation status of the FDFT1 gene region in the peripheral blood of AML patients enrolled in the TACL trial at baseline. P = .0021 across all 3 CpGs, by Fisher’s exact test. (B) Methylation status of the FDFT1 gene region in BM or peripheral blood (as available) from patients enrolled in the D-ADE arm of the DACOGEN202 trial at baseline. P = .0022. PD, progressive disease.
To broaden our understanding of the prognostic significance of the FDFT1 region, we re-examined the methylation data obtained from a separate phase 2 clinical study, DACO202.19 Children with newly diagnosed AML were randomized to receive a 5-day course of decitabine followed by cytarabine, daunorubicin, and etoposide (D-ADE), or cytarabine, daunorubicin, and etoposide only. Methylation analyses were performed using the same HM450 DNA methylation array. In the D-ADE cohort, 5 patients died due to refractory or relapsed disease. Therefore, we separated patients into 2 groups: alive in CR (n = 6) or deceased (n = 5). Similar to this study, at baseline, the FDFT1 region showed significant differential methylation between patients who were still alive versus those who died of leukemia (Figure 1B), suggesting that methylation of the FDFT1 DMR was associated with poorer overall outcomes in this independent clinical trial population. The difference in the methylation of this region did not correlate with the BM blast count or cytogenetics in either study (data not shown).
Investigating further, we noted that the region occurs in the first intron of the FDFT1 gene, a master regulator of cholesterol biosynthesis, and coincides with a chromatin immunoprecipitation sequence peak for the DNA-binding factor CTCF (a master regulator of genome and chromatin structure). We cannot yet unambiguously assign a functional role to this region.
The correlative biology study demonstrated expected decreases in DNA methylation post–AZA treatment in all patients. Importantly, we identified a single contiguous DNA hypermethylation region intronic to FDFT1, present at baseline only in patients with treatment failure, but not in patients who achieved CR. One limitation of this analysis is that the methylation analyses were performed on BM samples with variable blast percentages (median, 68.5%; range: 16%-94%). Fortunately, we were able to incorporate samples from the DACO202 study that tested decitabine, a different DNMTi, in combination with chemotherapy in de novo AML, because newly diagnosed patients usually have higher blast percentages, and the samples were enriched for blasts.19 The validation of our finding that FDFT1 intronic hypermethyaltion at baseline is also associated with worse outcomes in a different patient cohort is very encouraging, and suggests that the association between FDFT1 methylation and outcome is not limited to the relapsed patients. Recognizing the small number of patients included in both studies, we are currently prospectively investigating the significance of the methylation of this region in a randomized phase 2 trial of epigenetic priming that will include 200 children with newly diagnosed AML (www.clinicaltrials.gov #NCT03164057).
In summary, the phase 1 clinical trial demonstrates that AZA can be used safely in sequence with intensive chemotherapy to treat children with relapsed/refractory AML and offers encouraging clinical activity. Furthermore, DNA methylation in a region of the gene FDFT1 was highly associated with patient clinical outcomes. Further studies will be required to test the efficacy of this combination and to investigate the underlying biological mechanisms involved in hypermethylation of the identified region.
Acknowledgments
The authors thank the clinical trial participants and their families, the referring physicians and care teams, the trial site clinical research teams, faculty, and staff, and the TACL consortium operations staff.
This work was supported, in part, by the Gateway for Cancer Research Foundation, the STOP Cancer Foundation, Children’s Hospital Los Angeles, the Higgins Family Foundation, and National Institutes of Health, National Cancer Institute award P30CA014089.
Authorship
Contribution: W.S., T.M.C., and G.L. designed the study; W.S., J.M., P.G., R.S., H.B., A.E.P., Y.M., C.F., L.D.-P., A.S.W., and T.M.C. participated in the collection and assembly of clinical data, clinical data analysis, and interpretation; W.S., T.T., X.Y., B.S., P.J., L.G., T.M.C., and G.L. participated in the correlative biology study, data analysis, and interpretation; W.S. and T.T. wrote the first draft of the manuscript; and all authors participated in the critical review and revision of the manuscript and provided approval of the manuscript for submission.
Conflict-of-interest disclosure: P.J. is a consultant for Zymo Research Corporation. X.Y. is employed by Zymo Research Corporation. H.B. receives honoraria from Jazz Pharmaceuticals and is a consultant for Amgen, Jazz Pharmaceuticals, and Novartis Oncology. The remaining authors declare no competing financial interests.
Correspondence: Weili Sun, Department of Pediatrics, City of Hope National Medical Center, 1500 E. Duarte Rd, Duarte, CA 91010; e-mail: weilisun@coh.org.
References
Author notes
T.M.C. and J.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal